A Clean Bill of Health
James Finn describes the development of an award-winning setup for sterile filtration of APIs
WHEN you are dealing with the manufacture of an intravenous pharmaceutical product, sterility is key. A vital stage in ensuring this is the sterile filtration of bulk APIs (active pharmaceutical ingredients) and excipients (prior to their introduction into a sterile process train) to remove bacteria, micro-organisms and particulates which may be harmful to the patient. This is typically achieved using a filter with a nominal pore size of 0.22 µm.
When you are dealing with an antibiotic that is also on the World Health Organization’s List of Essential Medicines, being able to guarantee and prove that sterility, allowing for expeditious release of the final product, is vital to maintaining supply to critically ill patients.
Confirming the integrity of the sterile filters is key to ensuring process sterility. This is usually achieved by taking the filter offline, inserting it into a test rig, wetting the filter with either water or a solvent and then conducting a diffusion, bubble-point or a water intrusion test. These tests pressurise the filter with air, and monitor diffusion through to the downstream side of the filter. The rates of diffusion correlate to different pore sizes, allowing the integrity of the filters to be confirmed. To facilitate the test, the downstream side of the filter needs to have a route open to atmosphere, usually an open vent pipe, or a route back to the non-sterile test rig.
When you are dealing with an antibiotic that is also on the World Health Organization’s List of Essential Medicines, being able to guarantee and prove that sterility, allowing for expeditious release of the final product, is vital to maintaining supply to critically ill patients
As the filter usually needs to be positioned in an offline rig to perform the integrity test, integrity of the filter is confirmed prior to installing it in the process stream and running the pre-production steam sterilisation step.
Once the production process has been completed, the filter can be taken offline again and re-tested, post use, to confirm that the filter has not been damaged.
As the filter is tested prior to installation and steam sterilisation, the majority of batches are run with the risk that steam sterilisation may have damaged the filter. High pressure drops across the filter, or rapid changes in temperature, can affect the filter cartridge and create routes through the filter which would allow bacteria and micro-organisms through into the final batch. Damage is typically only discovered during the post-batch integrity test, resulting in a lost batch.
Ideally, an integrity test would be carried out after the steam sterilisation, as the final step before committing to a batch. As mentioned above, connections would be required on the downstream side of the filter, which would be considered sterile at this point. Opening this side of the filter up to atmosphere, or to a non-sterile test rig, would invalidate the sterilisation process as it would open up potential routes for contamination to enter the process stream.
Therefore, a major challenge of this post-sterilisation integrity test is how to ensure that the sterile side of the filter is not exposed to a non-sterile environment.
Equally important is the ability to repeat the operation of the filtration process, the methods of cleaning, steam sterilisation and the introduction of process fluids to the filters, and being able to remove human error. Discrepancies in how different batches are operated can affect the final product, which is why validating a process to be repeatable is so important in pharmaceutical manufacture. For a filtration step these discrepancies can be as small as different operators opening valves at different rates, resulting in different flow characteristics across the filters, or different temperature profiles across the system. This is where understanding the process, and automating as much of it as possible, in order to make the process consistently repeatable, and controlling the critical process parameters, becomes vitally important for a validated manufacturing process.
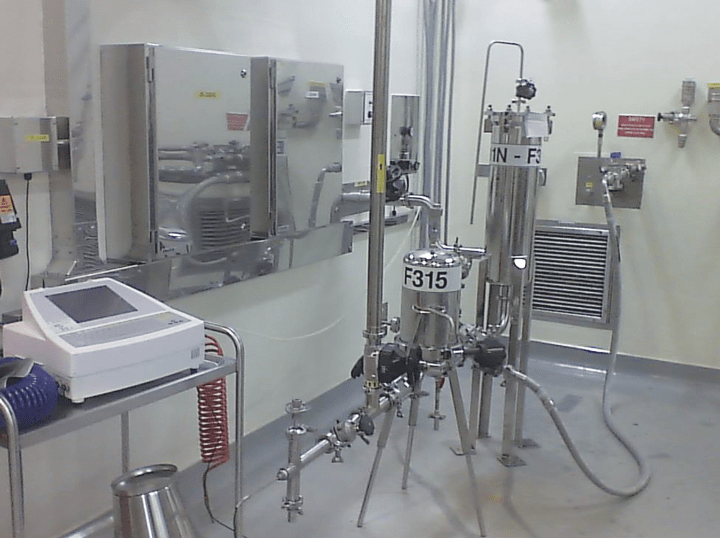
With this in mind, and as part of a much larger facility improvement project, a team was put together at GSK Ulverston, UK, in October 2015, to improve the sterile filtration steps of an antibiotic production process. The resulting filtration system that we designed changed the heavily manual operation, with a single sterile filter (see Figure 1), to a fully piped up, fully automated filtration unit, with two sterile filters in series, for added security, and integrated integrity testing with no routes for contamination.
The resulting design has achieved the following, compared to the original setup:
- it has removed all manual operations from the process, except for the replacement of the filter cartridges, allowing each batch operation to be run identically and removing operational discrepancies between batches;
- plant setup time has been reduced, with no offline testing, or manual setting up of process routes required;
- each operation is monitored to ensure critical process parameters are maintained, reducing processing risks;
- filter integrity and sterility is confirmed at the final point before introducing valuable product, eliminating costs of lost batches;
- as specific end points of the process are monitored, the processing operations are as efficient as possible, however, the monitoring will allow the process to be better understood and developed, allowing further process efficiencies to be realised in the future; and
- as the whole operation is now fully contained, operator and environmental safety has also been greatly increased.
There were a number of challenges, not all of them technical, that had to be overcome before we got to this stage.
Original scope
There were three rooms where sterile filtration took place within the facility. The existing process (see Figure 1) for each consisted of one sterile filter, approximately eight manual valves, and a selection of hoses and wall-plate connections allowing the desired process route to be set up.
Once a filter had been sterilised it was not possible to confirm integrity of the filter without exposing the sterile side of the filter to the room environment. So we were tasked with improving the sterility of the process, introducing dual sterile filtration, integrating the filter integrity testing, automating as much of the operation as we could, minimising the amount of equipment within the clean room, and retrofitting a new unit into an existing, congested, building – an interesting challenge!
The overriding design requirement of the process improvement was to design a system that would allow for integrity testing of the sterile filters, post sterilisation, with no risk of contamination. This would allow integrity and sterility to be confirmed as the final step prior to introducing a batch.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




