Vaccines: The End of the Cold War?
How an award-winning ensilication technology could remove the need to refrigerate life-saving vaccines
MOTHERHOOD changes people, irreversibly. For me it was not just becoming a parent, but it also influenced the direction of my research. When my daughter was only a few days old, I took her to the doctors to be vaccinated with BCG vaccine (against tuberculosis). The doctor took the vaccine out of the fridge and administered it directly. That’s when I asked: “why must vaccines be refrigerated?”, and then the natural follow-up question, “can I help to make them stable at room temperatures?”
From then on that second question became almost an obsession for me. At the time I was already a Royal Society University Research Fellow, working on developing porous alumino-silicate materials. I decided to dedicate some of my time to developing an idea – applying silicates (materials made of amorphous silica, SiO2) to the proteins within vaccines, to render vaccines stable at room temperatures. Silica is a great material, it is biocompatible, non-toxic, cheap, abundant and easy to manipulate.
An uncomfortable truth
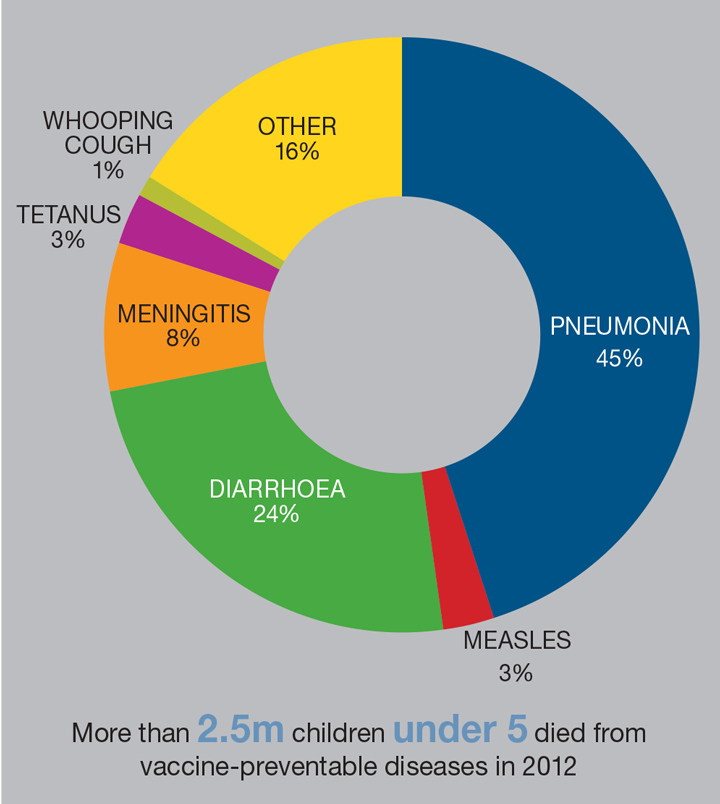
Why is this important not just for me but for people all around the globe? I suspected that even in the 21st century, when we have very effective vaccines, people are still dying from vaccine-preventable diseases. But I was wholly unprepared for the raw statistics surrounding vaccination. Despite the sterling job the WHO and UN are doing on vaccinating children around the world, there are still enormous problems. Thousands of children under the age of five still die every year from tetanus, measles, pertussis, rotavirus, and other diseases which are so easily prevented by vaccination. Low-income countries have orders-of-magnitude lower vaccination rates compared to developed countries.
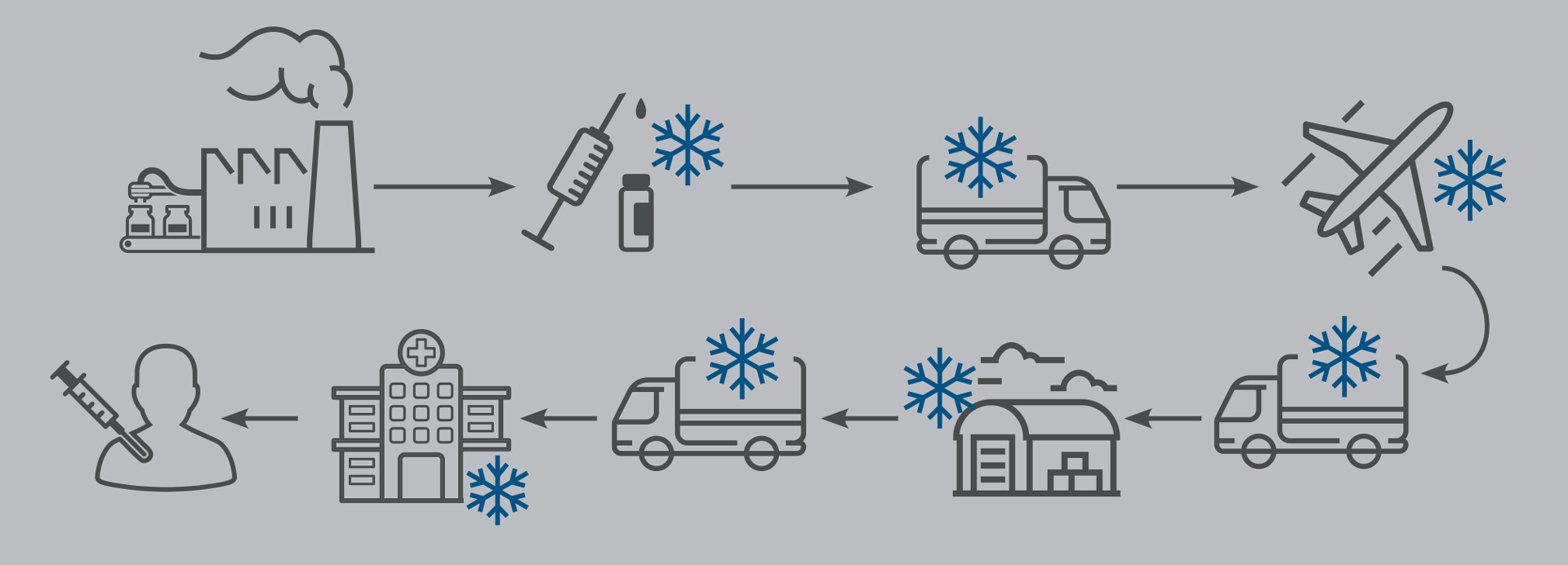
Why we are failing in protecting people, and in particular children? The problems with vaccine availability are very complex – involving conflicts, economic downturns, political issues, natural disasters, religious views, problems with physical delivery and their cost, to name but a few. Many of these issues make vaccine availability impossible in some regions around the world. Physical delivery is a particular problem because many vaccines spoil at temperatures above 8oC, as the proteins which make up the vaccine unfold and cannot re-fold back. Think of a boiled egg – once boiled it cannot be brought back to its raw state, because all proteins inside an egg unfold and tangle up while heated. Most vaccines also cannot be frozen as they would lose their potency; the ice crystals forming in the formulated liquid during freezing are also disruptive to protein structures.

This means that vaccines have to be stored and transported at temperatures of 2–8oC at all times – the so-called “cold chain”. In developed countries it is not a big problem, as there are specialised fridges and electricity available 24/7 allowing for this cold chain to be maintained in hospitals, GP surgeries, storage facilities and transport vehicles. In many developing countries, this is not the case as there may be no cold-storage equipment, or cold-storage transport. Sometimes there is no electricity, and roads and other infrastructure are inadequate to support the cold chain for vaccine transport and delivery.
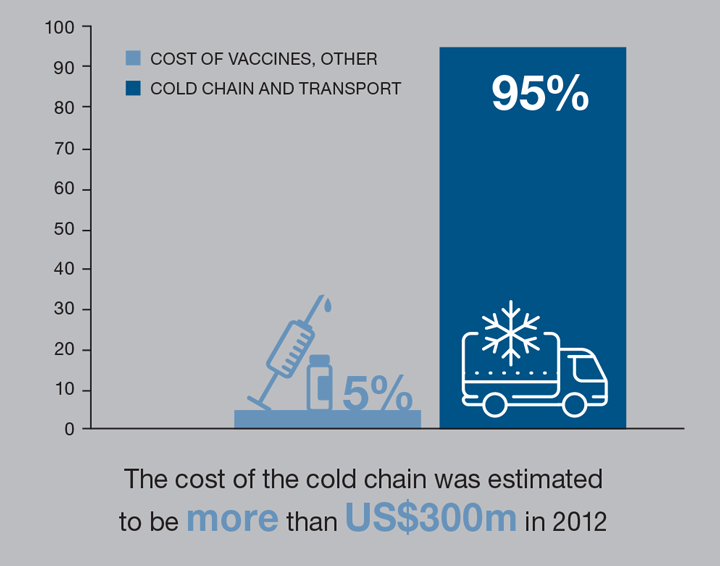
WHO estimates that anywhere between 30–50% of vaccines are discarded today because of cold-chain failures. Efficient alternatives to refrigeration would help us to make vaccines widely available
Because of problems with electricity and equipment, many vaccines are wasted. WHO estimates that anywhere between 30-50% of vaccines are discarded today because of cold-chain failures. It is also possible that some people in developing countries are receiving inactive vaccines because of cold-chain failures. The cold chain as a solution for vaccine storage and delivery was introduced by WHO in the 1970s, and it has remained unchanged for almost 50 years. Efficient alternatives to refrigeration would help us to make vaccines widely available. Even incremental improvements in vaccine coverage can have a dramatic effect on public health, as once coverage exceeds about 85%, “herd immunity” provides protection to unvaccinated individuals.
Thinking outside the box
The vaccines we have today are very effective and safe, but as mentioned earlier have complex issues surrounding their availability. I knew that I would not be able to change the politics or influence the economies or even people’s religious views. But I decided that there was something I could do. If we can make vaccines stable at room temperatures, without the need for the cold chain, I hope that will lead to wider vaccine availability.
Of course, the idea of mixing proteins and silica is not entirely new. There are many technologies today where silica is used as a support for enzymes. Most existing technologies make a silica shape – nanoparticles, nanocapsules, nanoporous monoliths and other shapes – and then add protein into that shape. This works well for applications such as enzyme catalysis or drug delivery, but does not protect the proteins from degradation, as they are free to unfold inside the pores that contain them. I thought what we need is something analogous to marine diatoms – tiny creatures living in seas and oceans – which grow a silica shell around their bodies to protect themselves from the environment. I decided that we should grow silica in a ‘tailored’ manner around the individual proteins to protect them from unfolding. The silica shell would mimic the shape and size of the individual protein and hence would act as a tailor-made coat to envelop the protein, rather than a pre-formed shell or cavity which contains the protein but does not conform to its shape.
Developing a crazy idea
How hard can it be to sell the idea of using silica to make a tiny shell around individual vaccine proteins to stop them from unfolding? When I first had this idea in 2010, around eight years ago, my biggest supporter in this idea was Stephen Wells (who happens to be my husband), and is currently working in the Chemical Engineering Department here at Bath. We have previously published many papers together, as I use his software for some of my studies on porous materials. Together we discussed this idea many times and looked at possible solutions. At the time I was working in Oxford, and I spoke about this to many colleagues. Although some said that it was an interesting idea, many of them shook their heads and said that it was an impossible solution, that it was a crazy idea, that I should stop wasting my time. What was apparent was that I wouldn’t receive any support among my chemistry and engineering colleagues at that time.
What I was really missing at that time was biological knowledge – I learned about proteins and vaccines by reading literature, but since I had never really worked with biological matter in the lab, I had no idea how even to start working on this. In 2013, I moved to the Department of Chemistry in Bath and discussed the idea with Paul Raithby (professor of inorganic chemistry) who thought it a brilliant idea and told me to go speak to Karen Edler in Chemistry, and Jean van den Elsen in Biology. So I did – and this was the turning point, because they provided me with TEOS (tetraethyl orthosilicate – a silica precursor we now use for ensilication), and “bucket loads” of cheap, easily-available proteins. A working lab was born.
Even incremental improvements in vaccine coverage can have a dramatic effect on public health, as once coverage exceeds about 85%, “herd immunity” provides protection to unvaccinated individuals
My first attempts (about 30 or so) were unsuccessful. I was ready to give up. As a scientist, I always doubted whether it would work or not. We have to apply critical thinking, especially on our own hypotheses. Looking back to those attempts I can see what the problems were, and it brings a smile to my face now to consider my early attempts – I knew practically nothing about proteins, had never worked with soft matter before, had a very poorly stocked lab because of the recent move, didn’t have students at the time, and had very little money for consumables and equipment. Nevertheless, I persisted. As a human being, as a mother, I wanted this to work. I will make this work!
I’ll Google that for you
Another milestone in the development of the ensilication process, for me personally, was an invitation to GoogleX’s Solve for <X> conference in California in February 2013 (http://bit.ly/2CuDS8m). GoogleX acknowledged that the cold chain is one the biggest global challenges in the world today, and I was one of only two people from the UK presenting at this conference, among 18 invited speakers. The other global issues discussed included use of nuclear fusion, space exploration, global warming, refugee settlements, and the nutritional value of crops in Africa. This was not a scientific conference, as it was attended mainly by Silicon Valley executives and investors, but it had a very positive impact on me personally. Speaking to all of those people made me open my eyes on other issues and look at my work on ensilication from a broader perspective. It also gave me more confidence that I am doing something bigger than just mixing chemicals in the lab. I think this was a very valuable experience for me as a person, to grow and see the problem in a wider context.

Our first successful ensilication happened the week after I came back from the GoogleX conference. When back in the lab, I decided to completely change the way we did it. I realised that this problem cannot be solved only by thinking as a chemist; I needed to think of all aspects of the ensilication process, as a chemical engineer, as a protein biologist, as a technician, as well as a chemist.
Some of the changes we made included:
a) we decided to prepare the silica first – our pre-hydrolysis step – where we strip the TEOS of its ethyl groups. This prepares the silica to interact with protein;
b) we changed and tuned all quantities of protein, pre-hydrolised TEOS and buffer;
c) we changed all glassware carefully to match the chemical quantities and processes; and
d) we changed all times for pre-hydrolysis and ensilication.
All of these changes were done as a result of careful observations and tweaks to make the final product in a powder form.
Once the protein and silica precursor are mixed, silica nanoparticles rapidly grow around proteins, and within seconds a cloudy suspension appears – this indicates that the nanoparticles are large enough to scatter light, and they start to precipitate. Once nanoparticles have precipitated, we can collect them. At this stage, a silica network covers individual proteins and protects them from unfolding. This powder can be stored without fridges for many months.
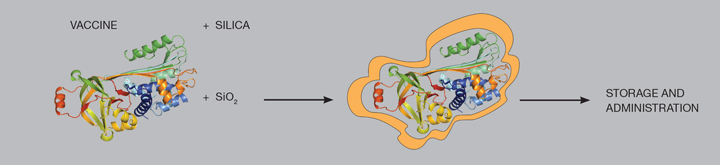
Proof of the silica pudding
To demonstrate that ensilication worked, we needed to show that the proteins are inside silica and that silica protects the proteins from degradation. It is not easy to detect the proteins inside silica – many chemical and engineering methods are not sensitive to proteins, while many biological methods are not designed for hard matter. We used many techniques to show that the proteins are completely enclosed inside silica, including experiments at large international facilities such as the Diamond Light Source and the European Synchrotron Radiation Facility. To show that silica protects proteins from degradation was a bit easier. We heated ensilicated powder with proteins inside to 100C, we soaked it in strong acid, we left it unprotected on the bench for months, then years. To my utter surprise, the proteins in silica not only survived after such harsh treatment, but were fully functional when released into solution once more. This was the time when I finally started believing that what I am doing will work.
Challenges
Ensilication brought many challenges for me. One of the biggest barriers was bridging the social and communication gaps between the chemists, engineers, protein biologists, pharmacists and immunologists who became my collaborators. These barriers sometimes were as easy to identify as a difference in language and a difference in experimental approaches. Sometimes they were a little more subtle – a “bucket load” is a very different measure for a chemical engineer, a biologist and a chemist. For me personally, building confidence was one of the keys steps in battling through criticism and colleagues’ scepticism at the beginning of the project and accepting the expert knowledge from colleagues in the areas where I am not an expert.
In the lab, the challenge is making sure that my students are working in a safe environment, as there are two streams of research happening in my lab today – one on vaccine ensilication and another on developing porous materials for catalysis applications. We still battle with the lack of equipment and consumables, but these are solvable problems.
Another large challenge was determining the correct scale to use for the process. When I started, I was using large quantities of materials. Once we started optimising the ensilication process for specific proteins, we sometimes only had milligrammes to work with, so the process had to be scaled down. This was not easy, as the scaling is non-linear and required much optimisation. While we are currently working on this smaller scale, in the future we will need to scale the process back up to industrial levels, presenting us with the opposite challenge.

What’s next?
I now have several students working on ensilication with me. My next steps will be to seek funding to continue the work and build relationships with vaccine manufacturers so that we can work together on making vaccines stable at room temperatures and working with vaccine clinicians to ensure safety. As a scientist I’m very excited to be looking forward to applying ensilication on other classes of biopharmaceuticals such as antibodies, enzymes, drugs and even perhaps cells.
In the future it will be beneficial to start talking to vaccine distributors for using ensilication as a replacement for the cold chain, so that we can transport and store vaccines without refrigerators.
It is exciting to be working on a technology which potentially will remove our dependence on the cold chain; which can make vaccines available for every person in the world; which will stop vaccine wastage; which can save millions of lives around the world in the future. As a mother, as a human being, I think saving even one life will be worth working towards.
In 2017 Sartbaeva was awarded the IChemE Biotechnology Award, and also the WISE World Award.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




