PPE Challenge Accepted
Zeb Ahmed and Nick Geary talk to Adam Duckett about the projects they are facilitating to help fight Covid-19
YOU’D have to have been hiding under a rock – and under the current circumstances who could blame you – to learn right here, right now that the world faces a pressing shortage of PPE. The coronavirus pandemic has disrupted supply chains and put healthcare workers at significant risk as many have been asked to continue leading the fight against infection without adequate protection.
While the supply of PPE is ramping up, it is curtailed by production bottlenecks, including the many months and millions of dollars required to set up new production lines for the non-woven “meltblown” polypropylene that acts as the life-saving filter in masks.
These circumstances have prompted volunteers on IChemE’s Covid-19 Response Team to search for ways to help bypass the shortage of fresh supplies: one that asks “can we use a barrier technology that provides the type of engineered isolation commonly seen in manufacturing environments?” And a second, “can we decontaminate used PPE rather than simply throwing it away?”
In isolation
Zeb Ahmed is leading an initiative on behalf of IChemE to help promote a barrier technology that was first demonstrated on YouTube by UK pharmaceuticals consultant Justin Mason-Home. The concept is relatively simple and would be familiar to those who work with isolator technologies that are used to protect workers from high hazard substances in drugs manufacture.
The device – called the Healthcare Worker Barrier Protection System – consists of a clear PVC barrier with integrated gloves that could be used to isolate testers from potentially-
infected patients.
“Take the UK’s COSHH regulations: the Control of Substances Hazardous to Health. Under any normal situation, PPE is the last line of defence,” says Ahmed, who explains that countries around the world mandate following a tiered hierarchy of controls (see Figure 1).
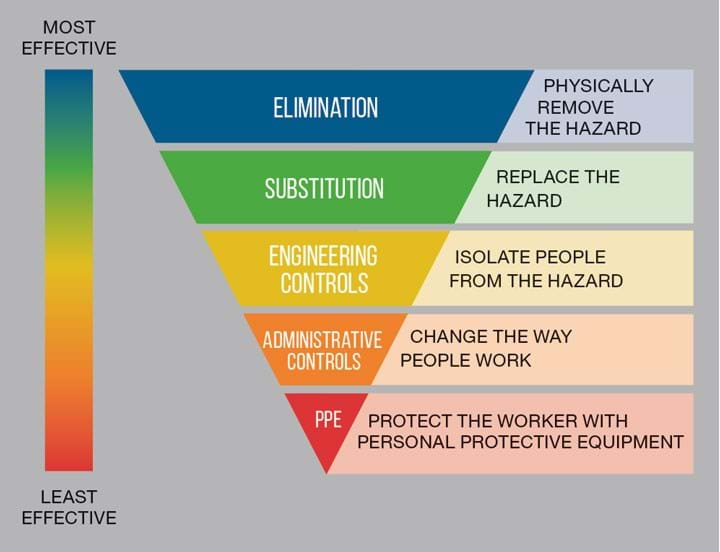
“The first thing you would do is try to eliminate the hazard. Obviously with Covid-19 we couldn’t do that. The second thing you try is to substitute it with something else. Obviously, we can’t do that either. So, then the next thing you try is a barrier – or engineering controls,” Ahmed says.
“If you look at the COSHH regulations this should be used before you go to PPE or administrative controls.”
The device, which would be made using CNC programming and RF polymer welding, has several variants. One includes fixing the barrier inside a doorway and could be used to separate medical staff from patients inside a GP surgery. It would cost £100–200 (US$120–240), has a lifespan of one year, and could be installed by a low-skill tradesperson in less than 10 minutes. The gloves are flexible enough to allow the user to perform all the necessary tests with a double glove system to provide additional protection.
In this scenario, the patient arrives on the “dirty side of the barrier”, collects a testing kit and hands it to the gloved hand of the healthcare worker who performs the throat swab or other medical procedures. The swab would then be put in a labelled container and the patient would then deposit it for analysis. The healthcare worker safely isolated on the “clean side” would then change the outer potentially-contaminated glove and then if necessary, use cleaning products within reach on the dirty side to sanitise any surfaces. Once complete, the next patient arrives and the process begins again.
“It gives a level of protection at low cost and is very simple to use and install,” Ahmed explains.
“The cost of PPE in a month could reach £13,000 if each time you saw a patient you had to change mask, gloves, and gown.”
Furthermore, the materials used in the barrier are different from those used in PPE.
“It’s a sustainable solution. You are not competing with the same supply chains or same raw materials.”
Another variant would better protect staff working at drive-through testing stations. Concerns have been raised about testers exposing themselves by leaning into patients’ cars to take swabs while wearing relatively little PPE. This system works much the same as the one above, but the barrier is fixed to the outside of a structure and concertinaed so it allows the tester to extend and retract the gloved barrier depending on the size of the car.

The device would help limit exposure, save on the use of PPE, reduce downtime between tests and therefore increase throughput. This means that there could be less reliance on military testers as seen in the UK, meaning tests could be carried out by trained healthcare workers, which should improve testing consistency and reduce the number of false positives, Ahmed says.
“The net effect is it will save healthcare worker lives because you are immediately curtailing the spread of the infection,” he adds.
The Covid-19 Response Team, including Ahmed, Adam Hawthorne and Hamza Jamal, helped develop the idea for submission to an EU funding competition called the EUvsVirus hackathon. They worked with architects from Bouygues Energies & Services UK to produce 3D conceptual drawings and videos showing how the device would work in practice.
“While the team did not win the competition, which included more than 9,000 participants, the inventor has now secured a field trial in a medical setting. Ahmed says the IChemE team is waiting on the next stage of results and is keen to continue helping by introducing the inventor to contacts and building awareness through international networks.”
Asked why authorities have chosen PPE over engineering controls, Ahmed said: “It’s understandable because we’re in an emergency situation, and we need to respond quickly. They probably didn’t realise that barriers could be deployed at scale, at low cost. Next time around, I think the barrier will feature more prominently than it has done in this first wave.”
Cleantech
While we wait for any future move up the hierarchy of controls, a project led by Nick Geary is looking to help transfer a technology to the UK that the US is already using to decontaminate used PPE. The Battelle Critical Care Decontamination System was developed in response to the SARS epidemic. It is a self-contained, mobile decontamination system that uses vapour-phase hydrogen peroxide (VHP) to sanitise masks for reuse. Each unit runs a 2-5 hour cycle and is designed to decontaminate 80,000 masks per day.
Geary explains that the vapouriser unit is about the size of a washing machine on wheels so it can be rolled in and out of a room.
“You pour liquid in and it vapourises the hydrogen peroxide, pretty much like a humidifier,” he says.
To complete the operation, Battelle hit upon using the simplest room they could find – a shipping container – to play host to their decontamination operation (pictured).
Geary used to work in the US close to where Battelle is based and through his contacts managed to arrange a call with the R&D organisation’s Vice President. He was told Battelle could provide a unit through its UK office but could not advise on how to operate it in line with UK regulations.

“The next day I got a phone call out of the blue from NHS England asking whether IChemE could help with this approach and would our members be able to facilitate the operation of one of these units should it be brought over.”
Geary secured support from the IChemE’s leadership team to proceed, but now weeks on, the NHS is still considering the idea. Geary says the delay is disappointing given the urgency, but the team has not been put off.
“We decided that rather than waiting we should push the project forward…from the bottom up and find a local hospital willing to work with us.”
The team consists of IChemE and partner institution members working with a consortium of UK companies - one of whom is running tests using a stand-in for the virus on unused PPE. This will help identify the cycle parameters needed to adequately kill the virus and help secure approval for use.
“Step one is gasification of the space; step two is change the parameters slightly so you get a micro-condensation [of hydrogen peroxide] on the surfaces of everything that it touches, like the fog in the morning that forms the dew. Then those micro-condensation droplets soak through the fabric of the mask so that the virus is inactivated regardless of whether it is on the surface or it is within the weave of the mask. And the last step, which is the most complicated and longest, is how to safely get all of that hydrogen peroxide off the mask so that it is safe for someone to use.
“It’s classic chemical engineering: diffusion and flow from high concentration to low concentration.”
Other considerations include: using a chemical or enzyme marker that ensures that the right amount of hydrogen peroxide has contacted the PPE; safety of the operators; ensuring a chain of custody using barcoding; and recording how many times each piece has been decontaminated. The US is cycling each item a maximum of 20 times, as while the decontamination process does not impact filtration performance, it does degrade the elastic fastenings.
“Phase two would be to find a partner hospital that could supply the contaminated masks and then we would help them to get a unit. Preferably we want to partner with a teaching hospital or a research hospital because a lot of those already have a decontamination room…and the ability to measure the concentration of pathogens.
“If anyone has a contact at an NHS Trust that they think could help please do email me to discuss: covid@icheme.org.”
Once the cycle has been shown to work, they would then help in the compilation of a report and share the results with regulatory bodies and healthcare worker unions to help secure necessary support for further rollout.
Asked how long it could take to see it being used in a UK hospital providing reusable PPE to staff, Geary says: “We think it should take no more than 30 days. The unknown for us is the speed of government approval.
“Obviously that approval process would need to be expedited and we are already in contact with key organisations to get an understanding of what is needed.”
Geary is hopeful that the authorities can move quickly given the UK’s quick approval of an antibody test from Roche, though he rues the time that has already been lost.
“Unfortunately, you and I are talking about a technology that the US, Canada, Finland and others have been using for over a month now.”
Geary and the team are not immune to the PPE shortage and have requested that anyone who can spare unused PPE please donate it so the team can help to ramp up testing. See p58 for more details on how to help.
And the final note here is that if IChemE volunteers can help facilitate the use of either of these technologies they would help alleviate shortages and the environmental burden of producing and disposing of so many single-use pieces of PPE.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




