Vaccine Development: Mind the Gap

Timothy Clayton details IChemE’s Covid-19 Response Team’s work on identifying what needs to be done
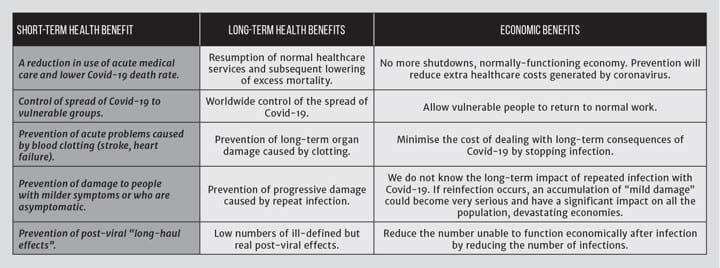
VACCINES are a wonderful invention that have transformed the health of billions. Not only can an individual be protected by a vaccine but, once enough people have been vaccinated, the whole population is protected because the infection cannot spread in a sustainable way (herd immunity). If a good vaccine that can generate an immunity to the virus causing Covid-19 is developed, there will be huge benefits (see Table 1).
We are promised a vaccine at record speed but Ken Frazier, CEO of MSD notes that we must be careful not to be too bullish about the speed of development of a new vaccine. In the last 25 years only seven truly new vaccines have been developed and taken to the market. Making a new vaccine is difficult. The fastest vaccine ever brought to market was for mumps, and took four years. Normally a vaccine takes about ten years to develop and costs more than US$1bn to get to market. Consequently, there is a timeline that allows for production facilities to be built and for supplies to be ramped up. This is not the case for Covid-19; everybody wants to get back to normal and a vaccine will be invaluable – but where is the capacity to make and distribute vaccine when a good candidate is available?
Development timelines are constrained by clinical studies. Because the vaccine is given to healthy people it is essential to ensure its safety using a large number of subjects. Before vaccines can be used in the general population, tens of thousands of people need to be included in clinical studies to test safety and efficacy. A major challenge in development of a vaccine is carrying out a clinical study that will be conclusive. The clinical trial must be carried out in an area where the virus is actively infecting a large enough number of people to allow demonstration of efficacy and duration of protection. A vaccine that lasts for less than a year is not much use to anyone. At present Brazil, South Africa and the US are being chosen for trials, but if the rate of transmission drops during the trials the data may not be conclusive enough and the trial period may need to be extended and new sites opened in other countries. When conclusive data are available, everyone will want the vaccine immediately.
As part of the Covid-19 response, an IChemE volunteer team has been looking at the options to help this extraordinary effort to develop and manufacture a vaccine.
The challenge
What process will be used? We want to know which vaccine will work, and can we make enough? Before major decisions on manufacturing capacity are made it is normal to answer three questions. Is the vaccine safe? Does it give immunity? And how long does the immunity last?
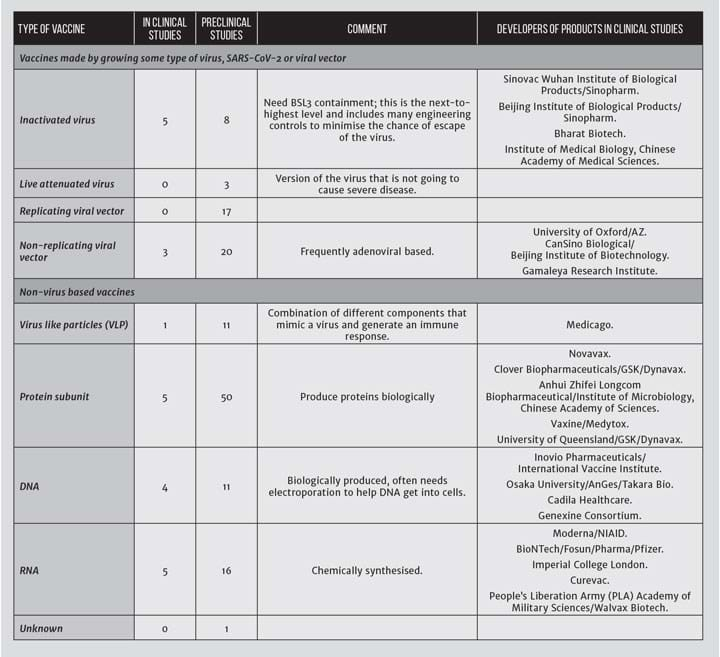
There are at least 160 candidates in development (summarised in Table 2). Many of the vaccine approaches being used have never produced a marketed vaccine.
There are significant differences in the methods used to produce different types of vaccine, so we can’t make general assumptions about what might be needed yet in terms of production capacity for drug substance or the type of filling and distribution capacity. For example, will the product be filled into multi-use bags, single or multi-use vials, pre-filled syringes or special devices, and will it be stored and transported as a liquid or lyophilised and will it be frozen, chilled or at ambient temperature?
Assuming that one, or several of these vaccines is successful, what strategy will be used to make it, and where? There is no clear definition of what a successful vaccine to prevent Covid-19 would look like, and questions remain. For example:
- Is a quick and dirty initial vaccine which is not as safe as would normally be accepted likely to be registered? Apart from liability issues (who will pay compensation and how much) there is a danger of losing the trust of the general population and generating a perception that the vaccine is not worth the risk.
- What is the acceptable response to the vaccine? (% immunised, length of immunisation, full immunisation vs partial immunisation –what if we mitigate the infection to reduce the death rate but don’t stop the spread?).
- Will the supply of the vaccine be limited by materials or delivery system costs/availability? (simple examples are availability of disposable bioreactors, cell culture medium, RNA manufacturing components, glass vials, silicon rubber tubing and syringes and needles. Some vaccines also need an electroporation device to get the vaccine into cells after injection.)
- How quickly will the supply need to be ramped up? We are adding a need for billions of doses of product that was not needed previously. A new vaccine plant with filling capacity will normally take 3–5 years to build and commission depending on location and capacity. We also need to continue other vaccine manufacture. Major vaccine manufacturer GSK claims to already make about 3m doses of vaccine a day. That is about 1bn doses per year – or about 1/15th of the quantity just needed for Covid-19 but not necessarily available.
- The race today is to make a vaccine – any vaccine – and cost is not a major consideration. However, as time progresses, governments will want a cost-effective vaccine. To date Moderna is thought to be targeting US$50–60 per course (US$25–30 per dose), Biontec/Pfizer US$19.50 per dose, and AstraZeneca a few dollars per dose. To use the Moderna vaccine for 1 cycle of vaccinations in the UK would cost about US$3.4–4bn just for the vaccine. Even a few dollars a dose will be out of reach for much of the world’s population. As the memories of the economic cost of our recent shutdowns recede, there will also be significant pressure to reduce annual re-vaccination costs if they are necessary, so a second round of more cost-effective vaccines may be needed.
- Allocation of vaccine - will there be enough to supply key populations?
- Will we have a choice of vaccines?
Primary objectives
Our team’s first objective was to find out what is the state of play for vaccines worldwide, but with initial focus on the UK and Ireland because we have the best knowledge of the UK and Europe. We set out to:
- determine if this is an area where IChemE can help and if we can see an immediate route to help;
- find out the state of play of candidates and develop contacts in this area; and
- based on state of candidates, look at manufacturing and scaleup and see if there are any major gaps that we can help to fill.
Outcome of gap analysis
With the exception of the Imperial College RNA vaccine, the emerging picture is that any vaccine that shows promise will be taken by a big pharma player or the state (such as China). In general, individual countries have little control. This also limits the impact that IChemE can have, but there were clear areas where we can help, particularly in education and training and longer-term policy development.
Too much choice and too little certainty
We just don’t know which vaccines may be successful, but there are three potential outcomes: no vaccine; one vaccine; or multiple vaccines.
If a vaccine is developed there will be a need to manufacture and distribute billions of doses, potentially on a yearly basis.
There may be a need to make an initial vaccine that is good enough, followed by a better vaccine that has taken longer to develop.
Many vaccines are biologics, scaleup and technology transfer are difficult, and the product is defined by the manufacturing process.
Manufacturing and distributing a vaccine will be a major task. At present we can’t predict which vaccine will be successful so don’t know what manufacturing process and capacity might be required. What is clear from a UK perspective is that we are not in a good place to make our own vaccines above those needed for routine vaccinations. This is also the case for many other countries.
Vaccine manufacture and development is an international business with strategic decisions made by pharmaceutical companies dominating the location and type of capacity worldwide. Decisions are made based on profitability and efficiency. As a result of mergers and acquisition (M&A) activity and the tendency to concentrate resources in focussed sites, the UK is not a hub for biotech and vaccine manufacturing.
Some capacity in the UK could potentially be used for manufacturing if not already fully occupied and if disposable technology could be used and live SARS-CoV-2 virus was not used.
We should not ignore veterinary vaccine capacity, but the impact on food production or companion animal health should not be underestimated.
The new Vaccines Manufacturing and Innovation Centre (VMIC) building has had additional funding to speed up construction and increase filling capacity 20-fold, with a completion date of mid-2021 now being proposed. Once fully commissioned, VMIC claims capacity of about 70m doses filled in 4 months, so about 9 months of manufacturing should meet UK needs for vaccine if one is available for them to fill and the filling capacity is appropriate for the vaccine.
Manufacturing capacity
Biopharmaceutical manufacturing is a worldwide enterprise with capacity in some countries around the world, and much of this capacity is already booked. Some small-scale capacity exists in the UK but may not be available (Cobra, Fujifilm, Lonza for example). Different organisations are already booking capacity for at-risk manufacture, Astra-Zeneca is using Oxford Biomedica and Cobra for manufacturing its viral vector-based vaccine not just for clinical supplies but to supply the market if it is successful in clinical studies.
The suitability of a site depends on whether it is already registered as a manufacturing site for development or marketed material. Any facility used for rapid manufacturing will need to be already in use as a biotechnology or pharmaceutical facility and will need to be modified to some extent. The facility must be capable of being operated to good manufacturing practice and appropriate biosafety levels (BSL), with BSL1 being the lowest and BSL4 the highest. To make inactivated vaccine, BSL3 will be required – this is not available in many contract manufacturing facilities and is difficult to retrofit.
Expansion of capacity may be possible in modular buildings, depending on process, especially if disposable technology is used.
Low-yielding processes may need high volume steel bioreactors, there are few in the UK but there is capacity in Europe including Ireland, Denmark, Switzerland, Germany, Spain. Further afield, there is capacity in the US and Asia. Availability of capacity varies wildly and it is common to book capacity several years in advance. Fujifilm in Denmark has announced a significant expansion, but this will take several years to perform.
For traditional vaccine approaches we may need to repurpose animal vaccine capacity.
Why focus on disposable technology for smaller-scale biotechnology activities?
There are a lot of assumptions based on the use of disposable technology to increase speed of scaleup and facilities build. A key factor is the large number of doses and potentially small volume of commercial production systems used for virus vaccines. As well as not needing to order, build and commission stainless steel tanks and bioreactors, many functions can be contracted out. For a rapid response there is the potential to increase speed by outsourcing supply for some buffers and media needs and switch between different products more quickly. The aim is to reduce capital cost (focus cost on modular, flexible facility for disposables rather than fixed equipment), however the facility becomes dependent on a complicated supply chain to operate. This is not suitable for all purposes and short-term expediency may have long-term impact when large-scale long-term needs are considered.
State of play
Two very different high-profile vaccine approaches are being tested by UK organisations; neither of these approaches has ever been taken to the market.
The Oxford adenoviral vector is already in clinical trials, and a large partner – Astra-Zeneca – is now involved, and Cobra and Oxford Biomedica are manufacturing the drug substance for the vaccine. A group at Imperial is testing an RNA vaccine encapsulated in a lipid. Trial batches were made by a US contractor.
GSK is collaborating with Sanofi on vaccines, making its adjuvant technology widely available, and is involved in numerous other Covid-19 vaccine projects. It has also agreed to buy a 10% stake in CureVac, a German RNA vaccine company.
A worldwide and permanent capacity increase for manufacturing and filling is needed. The whole supply chain for any new vaccine is going to be fragile, and challenged, not only by the sheer number of doses required but potentially by the conflicting demands of different projects
China is moving several candidates forward, mainly using traditional approaches and has built a new manufacturing facility in about 100 days.
A worldwide and permanent capacity increase for manufacturing and filling is needed. The whole supply chain for any new vaccine is going to be fragile, and challenged, not only by the sheer number of doses required but potentially by the conflicting demands of different projects. For example, there is overlap between capacity requirements for existing therapeutic proteins, new therapeutic proteins to treat Covid-19 symptoms, and subunit vaccines. Filling vaccine at-risk is a common approach which means filling the vaccine before the clinical study is complete, knowing that it will only be used if the trial is successful. This will expedite supply if trial vaccines are successful, but will block filling of other vaccines or therapeutics that may be needed.
Actions
Engineers are people who want to get their teeth into problems and make things work, and the vaccine issue is no different. The community stands ready to help, and several members of IChemE are already directly involved with the vaccine effort through their employment. For the rest of us who want to help as much as possible but can’t directly engage through our jobs, we have decided on the following actions that reflect our role as a learned society:
- Education and training for academic groups and small companies on the importance of manufacturability and technology transfer.
- Modelling best and worst cases for manufacture and distribution, including the whole supply chain, to see how the chances of success can be optimised and bottlenecks identified and overcome.
- Involvement with not-for-profit facilities (eg VMIC) and facilitation of accelerated build and commissioning.
- Building contacts with organisations such as Wellcome Trust, Gates Foundation, universities, and small biotechnology companies.
- Monitoring of vaccine progress.
- As the potential candidates become obvious we can assess what could be done with the limited capacity if the candidates are made available for manufacture.
- Longer-term we need to help shape the understanding and strategic vision for emergency response to future pandemics (whether this is by white paper, personal influence, trade bodies, modelling, press releases).
As the situation develops, the team will review the gap analysis to see where help can be provided. We have already started to define what training can be provided and the best way to deliver it. Technology transfer has been identified by many workstreams and is clearly a priority where we have the opportunity to meet the needs of several groups with one training package.
The IChemE Covid-19 response team working on Vaccines Drug Substance Workstream comprises Tim Clayton; Chris Davis; Julia Gordon; Ridwan Doba; Sijin Saji; Arsalan Afkhami; Peter Holly; Kieran Falvey; Kevin Lam; Nur Izzaty Badrol Hisham; Paul Riordan; Nooryesha Choudhury; and Keith Plumb (ad hoc).
Further reading
1. Sharpe, HR et al, “The early landscape of coronavirus disease 2019 vaccine development in the UK and rest of the world”, Immunology, Jul 2020 Jul, 160(3): 223–232.
2. https://hbs.me/31EkpAE
3. https://bit.ly/3gK8GGR
4. https://bit.ly/3h4LvY9
5. https://vimeo.com/436370787
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




