Small but Mighty
Noor Al-Rifai and Rene Holm look at the use of nanosuspensions as long-acting injectables
IChemE’s Pharma SIG series, Access to Pharmaceuticals in the 21st Century, discusses the challenges for engineers working in the pharmaceutical industry, how they impact on the access to medicines for patients, and how things could be improved. In Part 4, we look at using nanosuspensions as long-acting injectables (LAIs).
Women in developing countries live with severe health risks such as HIV and unintended pregnancies, often leading to the death of the mother or the new-born due to inadequacy of the healthcare system. Global public health organisations have recognised that treatments and preventions for these diseases are critical to improving health, with wider-reaching societal benefits.
Long-acting parenteral medicines that can last longer, withstand high temperatures and are easily administered can improve access to medicines
The challenges associated with access to medicines in developing countries include: a shortage of healthcare workers, poor transportation, hard-to-access healthcare facilities, and insufficient infrastructure (sporadic or absent cold-chain facilities and electricity). A potential solution to this problem is the use of long-acting parenteral medicines, that can last longer, withstand high temperatures, are easily administered, and place less of a burden on the healthcare system. Long-acting parenteral medicines maintain therapeutic drug concentrations for weeks to months1, a substantial improvement in duration of action compared to daily oral pills. Injectables are the most common type, administered either in the muscle or in the subcutaneous tissue, and drug release can take place directly at the site of action for a local treatment or at a systemic level after absorption into the bloodstream.
LAI goals
LAIs have revolutionised therapy for schizophrenic patients, who often struggle to remember taking medication, and therefore benefit from a longer-lasting drug that is taken less frequently. The goal now is to explore this technique for other therapeutic areas, including infectious diseases and contraception, to name just a few. The chemical engineering community can help with disease management as well as addressing the regulatory changes imposed by healthcare regulators on the pharmaceutical industry. This can be through the design of new long-acting delivery types that are easy to administer and distribute, and which can unlock improved pharmacological behaviour (even longer-lasting drug release). Chemical engineers can also employ their modelling skills to help improve the understanding of the manufacturing processes, the product stability, and the drug release rate.
Current industrial methods for producing long-acting suspensions predominantly involve top-down bead milling, but high-pressure homogenisation can also be applied commercially, whilst bottom-up methods are mainly in the research phase. Discovering, developing and deploying new processing and analytical technologies will help to manufacture products with enhanced properties, as well as make the product development cycles shorter and more efficient, overcoming obstacles to delivering these products to patients.
Types of LAIs and processing technologies
DRUG DELIVERY METHODS
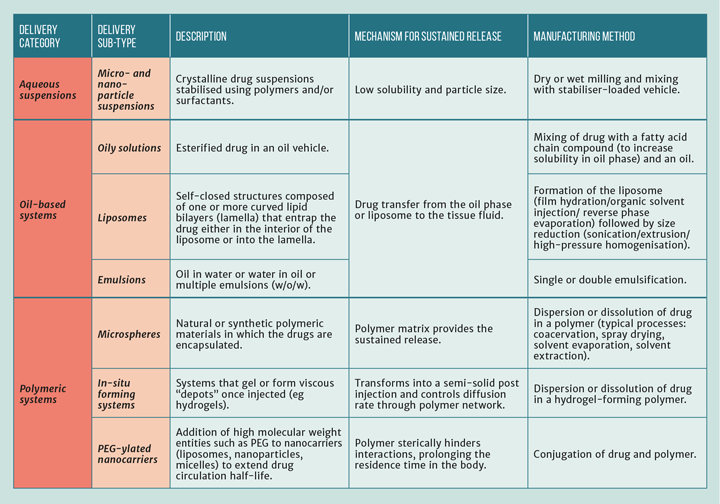
Long-acting injections and implants emerged as a sub-area of pharmaceuticals in the mid-20th Century and thrived, resulting in improved efficacy, tolerability and extended drug release. Capabilities to manipulate matter at the nano (~1 nm–1 µm) and micron (1–100 µm) scales with precision has led to the discovery of various drug delivery methods for long-acting applications (see Table 1). These delivery techniques can be used for small molecule drugs as well as large molecules (peptides and proteins). For vaccine antigens, micro- and nano-particles prepared from PLGA, poly(lactic-co-glycolic acid), are promising delivery systems and have the potential to eliminate the need for booster vaccinations. Beyond extending the drug release period, PLGA microparticles have also been shown to trap and retain vaccine antigens in local lymph nodes, thereby protecting them from proteolytic degradation1. This is a very exciting prospect indeed, given the current challenges imposed by Covid-19 to provide extended protection.
LAIs based on oil solutions have been used since the 1960s; however, crystalline particles in aqueous suspension for injection is a much newer dosage form. They have gained a lot of interest and most of the more recent products on the market use this technology; they work by letting the particle size and inherent properties of the compound control the release rate. The first two aqueous suspension-based LAIs were approved in 2009 (Olanzapine pamoate and Paliperidone palmitate), both for schizophrenia. Since then, more have been developed and many more are in the pipelines of different pharma companies. Aqueous suspensions have the huge benefit of offering the highest drug-loading potential, which allows more prolonged drug release time frames.
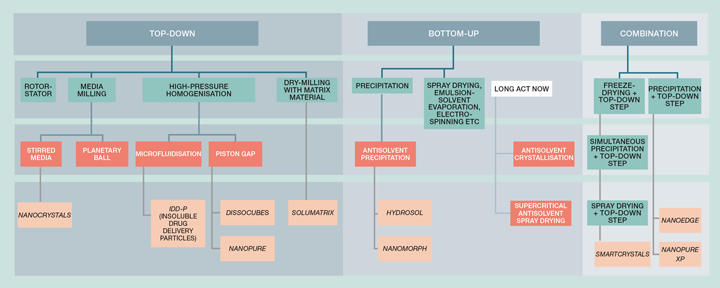
PROCESSING TECHNOLOGIES FOR MICRO AND NANO-SUSPENSIONS
LAI manufacturing technologies depend on the drug delivery platform and are summarised briefly in Table 1. For aqueous suspension-based LAIs, there are currently two main manufacturing methods: bottom-up (molecules to particles) and top-down (large particles to small particles). Figure 1 provides an overview of the different technologies for the production of micro- and nano-crystals via these two methods.
Top-down bead milling is most commonly used, followed by high-pressure homogenisation; the other processing methods exist only in patented literature, papers or as lab equipment. Bead milling is a popular choice for drug size reduction because it is relatively simple to perform, compared to other technologies, and is highly scalable and robust. However, it is energy intensive, and only a fraction of the applied energy goes into effective comminution. This creates:
- a heat transfer challenge due to high temperature generation;
- potential for product degradation due to mechanical or thermal stress; and
- grinding media and equipment wear (which can be avoided via careful material selection).
In contrast to top-down methods, bottom-up methods generate nanoparticles by building them from drug molecules and have the potential to tailor particles with unique nano- or micro-structures as well as eliminating the need for additional top-down processing. The synthesis of drug nanoparticles can be achieved by precipitation using an antisolvent or other means. The key to preserving the size of the precipitated particles is the use of excipients functioning as growth inhibitors, eg polymers or some other surface “poisoning” in order to prevent flux of additional molecules to the newly-generated nanoparticle surfaces and subsequent growth. This level of control is challenging to achieve, and process analytical technologies (PAT) will be essential to providing sufficient temporally resolved real-time measurements that enable determination of the processing endpoint (see Process Monitoring and Control later).
Development, manufacturing, and commercialisation challenges
The following scientific and operational challenges prevail for LAIs:
- Predictive modelling of manufacturability, product stability and pharmacokinetics. Modelling the eventual manufacturability, stability and drug release rate of compounds to allow differentiation amongst drug candidates pre-NME (new molecular entity declaration) in early development as well as support scaleup in late development.
- Novel drug delivery platforms and manufacturing methods. Engineering/controlling the physicochemical properties (particle size distribution, surface properties) to achieve the desired performance (in-vivo drug release profile and shelf-life stability).
- Process monitoring and control. Non-invasive and rapid analytics to allow in-process control without jeopardising product sterility.
Predictive modelling of manufacturability, drug product stability and pharmacokinetics
MANUFACTURABILITY
Regulatory guidelines for the pharmaceutical industry2,3 together with the promotion of quality by design (QbD)4,5, have provided a broad strategic perspective on how the pharmaceutical industry can improve its production processes and products6. The ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) guidelines also indicate areas where the demonstration of greater understanding of pharmaceutical and manufacturing science can create a basis for flexible regulatory filings (ie encouraging industry to demonstrate full understanding of the process, thus allowing easier transfers between scales and equipment following the drug approval). Chemical engineers have a direct role in meeting these needs via mechanistic modelling of pharmaceutical unit operations, helping to form fundamental scientific understanding, allowing optimisation, scaleup or transfer between equipment via scale and equipment agnostic performance parameters. On this front, efforts have been made to predict the processability of nanosuspensions both via mechanistic modelling as well as machine learning7, but there is still a lot to be done to fully simulate and predict processing outcomes, with the inclusion of material and formulation parameters that are relevant to pharmaceutical compounds and drug products.
DRUG PRODUCT STABILITY
Nano- and micro-particles in liquids are known to be thermodynamically unstable and have a tendency to agglomerate. To understand this behaviour, the physical stability needs months of trial-and-error experiments, which is time consuming and resource intensive. Enabling data science and ultimately predicting the stability behaviour of these complex systems requires large amounts of data with its associated context (physicochemical information of each component, processing conditions, and analytical results). Moreover, the drug product physical stability is not just a function of the formulation, but potentially the process parameters as well. To this end, molecular dynamics modelling has the potential to simulate effects under accelerated stability conditions by understanding miscibility, solubility and other physicochemical factors influencing product stability8.
DRUG RELEASE RATE
Better understanding of the influence of LAI physicochemical properties on drug release allows selection and development of the right compounds and formulations early in development, which can bring substantial benefits to drug development. A number of processes take place in response to an injected drug delivery system (eg dissolution, nucleation, permeation) influenced by the drug physicochemical parameters (eg solubility, diffusivity, molecular weight), as well as the physiological environment (eg pH, fluid volume). Not all of these events are well understood. Understanding and modelling these events may facilitate the selection of the right drug candidates, formulations, in-vitro methods, and animal models. Chemical engineers are skilled at modelling chemical and physical processes, and therefore can play an essential role in developing these pharmacokinetic models.
Novel drug delivery platforms and manufacturing methods
DRUG DELIVERY
Long-acting therapeutics are based on the principle of maintaining drug activity for long periods of time whilst being tolerable by the human body. Newer LAI drug delivery strategies aim to advance therapeutic drugs with features that include slow and controlled release, high efficacy, and tolerability. Examples of ways in which the drug delivery platforms presented earlier can be improved include minimising phenomena such as “burst release”, that can lead to unwanted side effects and limit the clinical application of LAIs. In addition, common excipients traditionally used for formulating tablets are often found to be unsafe or are immunogenic (induce an immune response) for parenteral delivery. Further research into these drug delivery platforms and excipients can help the application of this technology to a broader product portfolio.
Utilising the delivery device capabilities can bring about additional benefits. Devices that allow self-administration, can ease reliance on healthcare systems, thereby improving uptake of LAIs, particularly in developing countries. This can include disposable pre-filled syringes or self-administrable micro-needle patches that can deliver a safe and effective injection without the risk for cross contamination or error. Multipurpose prevention technologies offering combined prevention for several diseases and conditions (eg both contraception and HIV in one injection/device)9 can also simplify administration and resolve multiple issues at once.
MANUFACTURING
The manufacturing challenges for LAIs usually involve control over the drug physicochemical characteristics, both during manufacturing and during the shelf-life of the final drug product. Processes that can provide enhanced control or that can tune the drug to the desired in-human effect are highly desirable. For suspension-based LAIs, bottom-up synthesis of nanoparticles/suspensions opens up great opportunities for engineering nanoparticles to achieve certain product performance.
Several initiatives are currently in place to develop industrially-viable methods for bottom-up nanosuspension production. This would allow particle size fine-tuning without applying high energy size-reduction steps, potentially allowing more precise control over the drug characteristics and stability. One example is the LongActNow project (https://longactnow.eu) which is sponsoring five PhDs co-supervised by academia and industry to look into various routes for bottom-up nanosuspension synthesis: antisolvent precipitation, supercritical antisolvent assisted spray drying, as well as downstream separation steps required to produce a final drug product.
Process monitoring and control
Process monitoring and control of LAIs presents several challenges mainly due to the requirement for sterile processing, which puts a restriction on the number of samples that can be taken during processing and therefore limits the capability for in-process control. Other challenges prevail, imposed by the very fast processing timescales for bottom-up synthesis, and which can only be resolved by comparable analysis speeds.
Implementing PAT would allow non-invasive, continuous, rapid and real-time data acquisition, with much more process feedback than current offline measurements offer. However, in-line analysis in itself is complicated by the high solids loadings, (the often) sub-micron particle size, and variations in API and drug product characteristics through the process. These complications result in issues such as multiple scattering, possible particle interactions at high concentration, and particle phenomena occurring under flow.
LAIs are an important drug delivery platform... Chemical engineers can help by innovating on processing technologies and delivery platforms that can unlock different drug release behaviour and allow easier drug administration
To address these challenges, the consortium PAT4Nano (Process Analytical Technology Tools for Real-Time Characterization of Nano suspensions) – www.pat4nano.com – was established in 2020 and is a multidisciplinary consortium spanning industrial sectors (pharmaceuticals, catalysis, and inks), academics, research institutes, and PAT tool providers. The project has received a €5m (US$5.8m) grant from Horizon 2020 – the European Commission research and innovation funding programme – to develop and deploy (by 2023) PAT tools that generate continuous nano- and micro-particle size data, allowing more efficient and data-rich experimentation during drug development and manufacturing. Expanding beyond the measurements of particle size, realtime characterisation of other properties (eg polymorphic behaviour and rheology) would significantly amplify our understanding of LAI processing and control, and would be the next step towards real-time process evaluation.
Conclusions
LAIs are an important drug delivery platform for sustained drug release, with many advantages for patient adherence, efficacy and tolerability. Chemical engineers can help by innovating on processing technologies and delivery platforms that can unlock different drug release behaviour and allow easier drug administration. Developing mechanistic understanding and models that describe the impact of material properties and process parameters on the processability and product quality will make LAI production easier and more efficient, whilst PAT tools will help bring efficiency to the development work and flexibility towards life cycle management. These additional insights will hopefully help expand the long acting platform to a broader product portfolio and bring benefit to a broader patient population.
References
1. Burgess, D, Wright, J (Eds), Long Acting Injections and Implants, New York: Springer; 2012
2. US FDA, Critical Path Initiative, (2018), https://bit.ly/3lVF6DF
3. US FDA, Guidance for Industry, PAT – a Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance (2004), https://bit.ly/3lUQsb2
4. US FDA, Pharmaceutical CGMPs for the 21st Century – a Risk Based Approach (2004), https://bit.ly/3xBwzbd
5. US FDA, Submission of chemistry, manufacturing and controls information in a new drug application under the new pharmaceutical quality assessment system; notice of pilot program (2005), Federal Register 70; 40719–40720
6. Van Buskirk GA et al, Best Practices for the Development, Scale- Up, and Post-approval Change Control of IR and MR Dosage Forms in the Current Quality-by-Design Paradigm; AAPS PharmSciTech 15; 665–693
7. He, Y et al, Can Machine Learning Predict Drug Nanocrystals?; Journal of Controlled Release (2020), https://bit.ly/3fQjYek
8. Han, R et al, Predicting Physical Stability of Solid Dispersions by Machine Learning Techniques; Journal of Controlled Release (2019), https://bit.ly/3yFIjem
9. Krovi, A et al, Advances in Long-Acting Injectables, Implants, and Vaginal Rings for Contraception and HIV Prevention; Advanced Drug Delivery Reviews (2021), https://bit.ly/3CBCW25
This article is part of an ongoing series on Access to Medicines, developed in collaboration with IChemE’s Pharma Special Interest Group. Read the series here.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




