H2 and NH3 – the Perfect Marriage in a Carbon-free Society
TRANSITIONING our energy economy away from fossil fuel dependence towards one based on renewable and alternative forms of energy requires novel solutions for energy storage, in which the role of hydrogen has promising potential. The intermittency and seasonal variation of solar and wind power leads to a mismatch between energy supply and demand, which will intensify as we decrease our dependence on traditional gas and coal-powered generators. This challenge has driven extensive research into battery, capacitor and chemical energy storage as buffer systems to balance the variation of renewable energy supply on the grid.
As detailed in a previous article of this series1, a significant obstacle to the wider implementation of hydrogen in energy trade is its costly and energy-intensive storage coupled with safety concerns associated with its high flammability. In this article, we focus on the chemical storage of hydrogen in the form of ammonia to alleviate hydrogen’s storage and safety issues. Ammonia is explored as a complementary future energy vector with applications in specific cases.
Hydrogen carriers
Hydrogen-enriched compounds which are liquid at mild conditions, such as ammonia, methane and methanol, have recently gained attention as a distribution medium or for storage of hydrogen. Gaseous hydrogen storage requires high pressure vessels of up to 70 MPa while liquid storage needs cryogenic tanks maintained at -253°C. Compared to conventional fuels, hydrogen has a low volumetric energy density in both gas and liquid form.
In contrast to other forms of chemical storage, ammonia is the only carbon-free hydrogen carrier and can be synthesised from renewable sources as demonstrated by the opening of a pilot plant by Siemens in Oxfordshire, UK in June 2018 and a “green ammonia” plant by Nel Hydrogen and Yara starting up in Western Australia this year. In these projects, ammonia is produced by combining hydrogen from water splitting with nitrogen from the air. The ubiquitous abundance of nitrogen in the air – as opposed to carbon - supports the use of a carbon-free hydrogen carrier for a future, large-scale and sustainable energy storage cycle2.
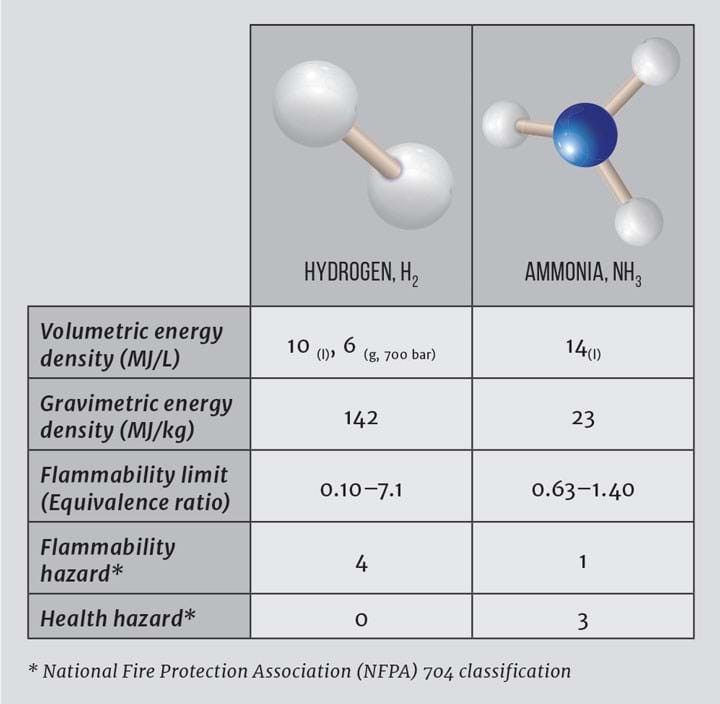
Ammonia is a promising hydrogen carrier owing to its high hydrogen content (17.65 wt%), established distribution network and ability to be liquefied at 10 bar or -33°C. Key properties are provided in Table 1. Hydrogen can be released on demand from ammonia through catalytic decomposition and consumed in a proton exchange membrane (PEM) fuel cell3. Alternatively, ammonia can be combusted directly or used in an ammonia-
fed fuel cell. However, each of these conversions carries an energy penalty.
While ammonia has a narrow flammability range and its toxicity is a concern, its strong smell is, however, useful for identifying leaks. To overcome the issue of toxicity, ammonia can be stored in metal salts and released reversibly at around 250°C4.
Practical assessment of H2 and NH3 as energy carriers
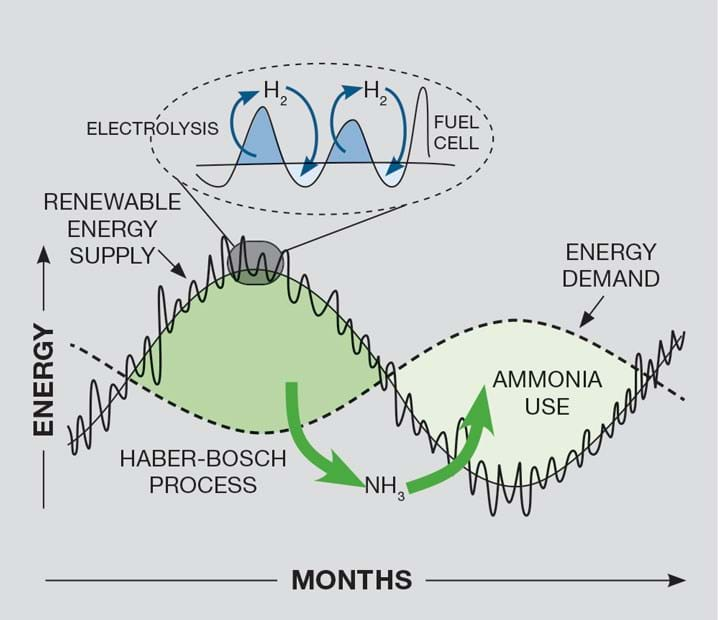
The potential energy applications of hydrogen and ammonia can be broken down into the following timescales and sizes: short-term energy storage; long-term energy storage; long distance transport/trade of energy; and fuelling the transport sector. While each category is likely to involve a combined solution, there are aspects where hydrogen or ammonia are inherently more suitable, as depicted in Figure 1.
Short-term (hours) energy storage
To match daily or hourly fluctuations in both supply and demand, hydrogen is a more promising option because the cycle of storing and harnessing energy in ammonia is unable to cope with rapid changes. The modern Haber-Bosch process which synthesises ammonia takes hours to days to reach steady-state, making it completely unsuitable for short-term storage.
Alternative ammonia synthesis technologies, such as electrochemical or non-thermal plasma-assisted ones, while more promising for rapid supply changes, are currently hampered by poor energy efficiencies and very low reaction rates due to simultaneous hydrogen evolution. Therefore, hydrogen is more competitive than ammonia for short-term energy storage.
Long-term (weeks–months) energy storage
An even larger scale of energy intermittency occurs on longer timescales – particularly between warm and cold seasons. For indoor heating to be powered by renewable energy, fluctuations in both weather and seasonal renewable energy supply need to be accommodated. Currently, reserves of natural gas are stored in the UK for heating during the winter months in the event of particularly cold periods.
Ammonia has a significant role to play in long-term energy storage due to its most advantageous feature – high volumetric energy density. When a large amount of energy needs to be stored for a long period of time, liquid ammonia becomes much more competitive due to the comparable volumes required for hydrogen gas storage and the energy losses (>30%) when storing liquid hydrogen5.
Liquid hydrogen is estimated to be at least ten times more expensive to produce and store than liquid ammonia due to the requirement to reach very low temperatures. However, salt caverns, although geographically limited, provide the possibility of storing a large amount of gaseous hydrogen at a cost 10% less than liquid ammonia5. Alternatively, various solid hydrides and hydrogen adsorbents are being explored to increase the volumetric storage density, but ammonia is still very cost competitive.
Long distance transport/trade of energy
As renewable energy begins to be adopted worldwide, it has become clear that some countries benefit from more renewable energy potential than others. A similar situation developed in the era of fossil fuels, when countries with natural stores developed major export industries. Unfortunately, it is generally much easier to transport fossil fuels than renewable energy (electricity) over large distances, but converting renewable energy into a dense vector such as liquid ammonia may be a feasible – and carbon-free – solution.
Recently, Australia has been exporting energy to Japan in the form of liquid hydrogen due to the politically-driven large campaign for hydrogen fuel in Japan, however Australia has recognised liquid ammonia as perhaps a more promising alternative in the long-term6.
When it comes to transporting either liquid ammonia or liquid hydrogen, ammonia has an additional advantage due to its already ubiquitous presence in world trade.
Synthetic ammonia has been used for over 100 years as a fertiliser and in order to feed approximately 50% of the world population, its current production exceeds 170m t annually. Its distribution from centralised plants fed by natural gas or coal is enabled by barges, rail cars, and pipelines to create a worldwide market exceeding US$5bn in 20177.
A particularly interesting aspect of ammonia transport is long-distance pipelines. The Magellan and NuStar pipelines in the US transport liquid ammonia from the coast and natural gas resources to fertiliser intensive regions over thousands of kilometres. When applied to energy transport, ammonia pipelines, rails, and trucks lose less energy than electricity transmission lines when transported over large distances8, making ammonia a promising medium for an international trade of renewable energy.
Fuelling the transport sector
The powering of automobiles, trucks and buses constitutes a major segment of energy consumption that needs to be displaced by renewable energy. Due to space, weight and cost constraints involved in transport, the high volumetric energy density of liquid ammonia makes it particularly attractive. However, the technological issues associated with harnessing ammonia’s energy, discussed in the next section, currently impede its use to fuel transportation. Therefore, hydrogen is currently a more feasible route to fuelling the transport sector, particularly with regards to large commercial vehicles where space and cost are less of a constraint, while small consumer vehicles may be more easily powered, at least in the time being, with batteries.
Ammonia – challenges and options
Synthesis of sustainable hydrogen
To store renewable energy in ammonia, as depicted in Figure 2, nitrogen can be obtained from air and hydrogen from electrolysis rather than methane. The Haber-Bosch loop compressors can be powered directly by electricity rather than steam, increasing their efficiency from ~45% to ~95%2. However, the hydrogen production efficiency from renewable energy via water splitting is significantly lower than from fossil fuels (ie reformation of methane), highlighting the key importance for the future development of efficient electrolysers to produce green hydrogen and hence green ammonia.
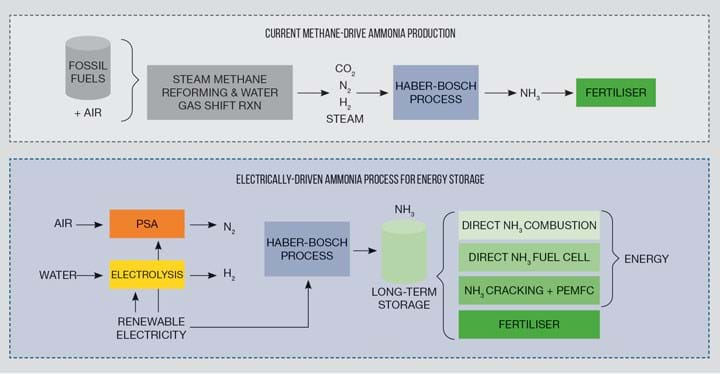
Redefining the Haber-Bosch process
Aligning ammonia production with renewable energy also requires re-defining the currently energy intensive Haber-Bosch process into one that is more agile and of low capital to match intermittent and isolated renewable energy. The high pressures (>100 bar), high temperatures (>400°C), heat integration and a large recycle loop of the process make it only operational at steady-state in large-scale plants. Redefining the Haber-Bosch process could be made possible through the discovery of new catalysts – which has been an active area of research for over 50 years – or through the use of a new ammonia separation technique (ie absorption) to replace condensation and overcome the equilibrium limitations2.
Harnessing energy from ammonia
Of the several ways to harness ammonia’s energy, combining the catalytic decomposition of ammonia with commercial hydrogen PEM fuel cells is an attractive option given the technological maturity of hydrogen fuel cells compared to ammonia ones. A key challenge, however, is the need to selectively remove hydrogen to prevent unreacted ammonia from poisoning the fuel cell catalysts. In addition, this process relies on developing catalysts which crack ammonia at conditions aligned with those of PEM fuel cells, as identified by the US Department of Energy, leading to recent extensive research in the field3.
Direct use of ammonia as transport fuel rather than hydrogen would be significantly cheaper when using a direct ammonia fuel cell, but this technology is in an early development stage. Alternatively, ammonia’s energy can be harnessed directly by combustion. This approach suffers from high nitrogen oxide (NOx) emissions, which has initiated research into low-NOx ammonia combustion. Humidified blends of ammonia and hydrogen have demonstrated low polluting flames and efficiencies competitive with natural gas gas turbines. However, further work is required regarding the reactivity of unburned ammonia and water as well as scaleup to industrial conditions9.
Round-trip efficiency
In the future implementation of ammonia in energy trade and storage, a key aspect is the round-trip energy efficiency - taking into consideration the energy required to synthesise ammonia from excess renewable energy and its delivery on demand. Currently, the round-trip efficiency of liquid ammonia is 11-19%, which is similar to the values of liquid hydrogen of 9-22%10. Technological advancements in electrolysis and hydrogen fuel cells will have an impact on both the viability of hydrogen and ammonia as renewable energy storage mediums and vectors, whereas improvements to ammonia synthesis and decomposition, combustion and/or fuel cells will make the use of ammonia more competitive.
References
1. Bimbo, N, “The Unbearable Lightness of Hydrogen”, The Chemical Engineer, 2019, https://bit.ly/3czr5V0
2. Smith, CJ, Hill, AK and Torrente-Murciano, L, “Current and future role of Haber Bosch ammonia in a carbon-free energy landscape”, Energy & Environmental Science, 2020(13): p331-344.
3. Hill, AK and Torrente-Murciano, L, “Low temperature H2 production from ammonia using ruthenium-based catalysts: Synergetic effect of promoter and support”, Applied Catalysis B: Environmental, 2015, 172-173: p129-135.
4. Klerke, A, et al, “Ammonia for hydrogen storage: Challenges and opportunities”, Journal of Materials Chemistry, 2008. 18: p2304-2310.
5. ACIL Allen Consulting, Opportunities for Australia from Hydrogen Exports, Australian Renewable Energy Agency, 2018.
6. Service, R, “Ammonia - a renewable fuel made from sun, air, and water - could power the globe without carbon”, Science, 2018.
7. Simoes, AG and Hidalgo, CA, The Economic Complexity Observatory: An Analytical Tool for Understanding the Dynamics of Economic Development. Workshops at the Twenty-Fifth AAAI Conference on Artificial Intelligence, 2011, https://bit.ly/2AsYItl
8. The Royal Society, Ammonia: zero-carbon fertiliser, fuel and energy store. Policy Briefing, 2020, https://bit.ly/2WXoa1t
9. Guteša Božo, M, et al, “Fuel rich ammonia-hydrogen injection for humidified gas turbines”, Applied Energy, 2019, 251.
10. Giddey, S et al, “Ammonia as a Renewable Energy Transportation Media”, ACS Sustainable Chemistry & Engineering, 2017. 5(11): p10231-10239.
This is the 20th article in a series discussing the challenges and opportunities of the hydrogen economy, developed in partnership with IChemE’s Clean Energy Special Interest Group. To read more from the series online, visit the series hub
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




