A Virtuous Circle: Chemical Looping has Solid Potential for Optimising Processes
Ben Anthony and Paul Fennell explore the advantages of chemical looping and call on governments to support its scale up in order to bolster green industry
Many processes which use or produce gases have the potential to be significantly intensified if they are adapted to use the technique of chemical looping. This technique could reduce energy losses and facilitate reactions which would not otherwise be feasible. This huge and varied promise, however, requires government to step up to allow engineers to scale the promise to reality. It could even advance the UK Government’s stated aim to make the country a “science superpower”.
Chemical looping is a suite of technologies which use a solid carrier material to transport a compound (frequently oxygen or CO2) from one part of a chemical process or fossil fuelled power station to another, in a cyclical manner. It can be used in many different guises – such as to produce electricity or hydrogen – and has excellent synergy with cement production and processes requiring hot oxygen. In most applications it is oxygen that is carried around the system, so an oxygen carrier is used, which avoids or reduces the use of cryogenic oxygen separation. Below, we will mainly discuss oxygen-based chemical looping systems, before briefly touching on other cycles of interest.
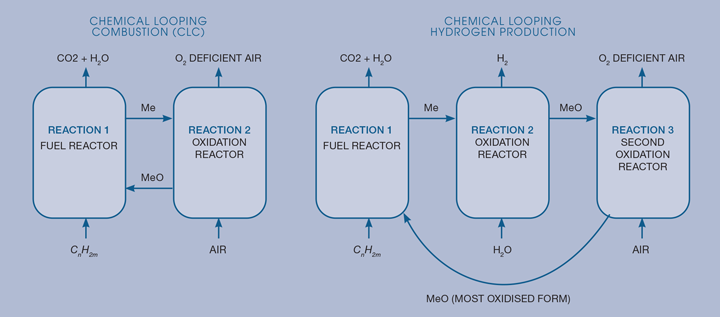
Figure 1a shows the basic layout to produce electricity – essentially, oxygen is sequestered into a carrier material from the air and carried to a fuel, where the fuel is combusted by the oxygen, which regenerates the oxygen carrier, which can then be passed back to the air reactor. Such a cycle produces a pure stream of CO2 that can be sequestered, heated to raise steam for power generation, and is an inherently low NOx system, as it avoids nitrogen fixation from the air. Many different processes can use similar looping cycles. In general, such processes take place at high temperatures, 500–1000oC, so they are frequently referred to as high temperature looping cycles. Figure 1b shows a hydrogen production process, where a fuel is used to reduce iron oxide (Fe2O3) down to iron, which is then oxidised back to Fe3O4 using steam, before the loop is finally completed by regeneration to Fe2O3.
Carry the load
The oxygen carriers are key to the process, and significant work has been done to develop them. They have to react in the correct way to enable a cycle to occur, meaning they need several oxidation states. Thus they allow the oxygen to be transported to the fuel, which then reacts with the oxide, reducing it to a lower oxidation state. To complete the cycle, the reduced oxygen carrier must then be regenerable back to its higher oxidation state. Only a few transition metals exhibit these properties, and of these iron, copper and nickel are the most important. Next, the temperatures at which the reactions occur must enable a sensible cycle to be set up.
The materials used must be robust to significant numbers of cycles – 500 is a frequently suggested number. The more expensive the material the more cycles it must undergo without crumbling to a powder or deteriorating in activity. The materials must be robust to any expected contaminants in the system (if coal is being used, for example, they must be resistant to sulphur), and as described above, they must be affordable.
Chemical looping is typically performed in a fluidised bed reactor, so the carrier particles must be dense enough to withstand this and not agglomerate readily. Finally, the materials must not be toxic, detrimental to the environment, scarce or prohibitively expensive, such as cobalt. Nickel, for example, is very promising for all the requirements above, but as a suspected carcinogen, it is unlikely to be developed outside a laboratory.
The materials must also not offer surprises. For example, in Canadian experiments exploring fuel cracking in a 1 MW(t) reactor that used fairly dense NiO particles, the 1 Hz cycles in the fluidised bed damaged the walls of the building, entailing construction work. Moreover, the materials need to be be recycled easily; and preferably regenerated in reactivity. For example, Paul Fennell’s laboratory at Imperial College has explored cycles which end up with the carriers being purged in a useful form – for example as particles of directly reduced iron, which can then directly supplement scrap steel use in an electric arc furnace.
Start of the loop
The original idea comes from work to produce pure CO2 from hydrocarbons, patented by Lewis and Gilliland in 1954. In 1968, Richter and Knoche, discussed it for improving and controlling combustions. Subsequently, the idea was explored in Japan using thermogravimetric analysers. It was also discussed by Lyon and Cole, in a paper with the intriguing title “Unmixed combustion: An alternative to fire” published in Combustion and Flame1. However, the first pilot plant demonstration occurred in Sweden, under the direction of Anders Lyngfelt, who subsequently worked with researchers in various laboratories in Spain, Austria and Germany, and has carried out thousands of hours of tests on different chemical looping materials.
Current laboratory scale research involves improving particles, accelerating reaction kinetics (perhaps by doping or otherwise altering the chemistry of the particles) and tailoring porosity to improve diffusion through the particles and increasing effectiveness factors for the reactions. Supporting the active oxygen carrier materials on inert compounds such as alumina can improve the mechanical properties (reducing attrition and fragmentation), chemical properties (by catalysing reactions) and physical properties (by improving particle porosity and reducing sintering) of the carrier particles. However, there is always a balance to be struck – the more inert material you have in your particle, the less active metal there is to react.
In research, there is a great desire to move beyond the small, 1–2 MW(t) pilots that have been developed to date, to larger demonstration scale. Unfortunately, the technology has been stuck in the “valley of death” between pre-commercial pilot and commercial demonstration for around a decade, as plans such as those of Endesa, to develop large scale processes failed to appear after the global financial crisis of 2008. Indeed, the output of the very successful CaOling project at La Pereda power station in Spain (which ended in 2013), was a conceptual design for a pilot plant at the 20 MW scale which was never pursued further despite the sterling efforts of Carlos Abanades and team at INCAR2.
It all goes round again
However, the general resurgence of climate change abatement technologies in the last few years, enabled by the Paris agreement, as well as companies beginning to accept that it is no longer acceptable to belch CO2 into the atmosphere, has led to some technologies being reconsidered. While Lyngfelt and Lecker claimed3 that there is no technological barrier for building a 1000 MW(t) plant, no such plant has been built, even at more modest levels around 10 MW(t).
Engineers agree that chemical looping is potentially enormously flexible and can be combined with gasification schemes, reforming schemes and, in an interesting variation, as a source of gas phase oxygen in a process known as chemical looping with oxygen uncoupling. Additionally, chemical looping can be used for chemical production (eg, ethylene oxide). There are also variations of chemical looping in which the solids may be partially heated by solar energy or used for energy storage.
Advantages and disadvantages of chemical looping
A major advantage of chemical looping is that it employs fluidised beds, although fixed bed versions are available. Fluidised beds are extremely versatile, and can be scaled from the 1 MW(t) to the 500 MW(e) level, and boiler companies have substantial experience in building them. Chemical looping combustion should be able to efficiently combust solid fuels, including biomass, offering a potential route for negative emissions (the so-called biomass energy with combustion capture and storage, ie BECCS). It is also easily compatible with hydrogen production, and Stuart Scott, Ewa Marek and colleagues are directing substantial and elegant work on this at Cambridge University. A particularly exciting direction for heavy industry integrates chemical looping with cement and iron production. In these processes, a bleed stream takes solids (either calcium oxide or reduced iron) from one combustion reactor and feeds it directly into either cement or steel manufacture, thus avoiding the necessity of the material to survive large numbers of cycles, and improving the efficiency overall.
In all cyclic processes, including amine scrubbing (the long-established use of organic amines to remove CO2 and H2S from waste gases), carrier materials do not last for ever and must be replaced, unless a bleed stream is used, as explained above.
There are also opportunities to use cheap natural ores as carrier materials. Recent successes here include work by Peter Clough and Ben Anthony’s groups at Cranfield, and by Tobias Mattison and Patrick Moldenhauer’s teams at Chalmers University in Gothenburg. These groups together have developed AI methods to select such ores.
No improvement without experience
Disadvantages of such processes include their complexity and lack of experience with the overall process. In the absence of full-scale demonstrations, it is impossible to guarantee that there will be no surprises. Failures with pressurised fluidised beds and coal gasification come to mind; neither produced sustained commercial technology. This history of failures and partial successes means that it is not easy to obtain bank guarantees and insurance for such processes. It is also important to realise that such processes are unlikely to compete with the efficiency of gas-turbine processes, and instead offer different market processes.
Pilot plant work in both atmospheric and pressurised versions of chemical looping at centres including the Universities of Utah and Darmstadt, Chalmers, and Canadian energy technology research specialist CanmetENERGY, all show strong potential. Demonstration projects at or above 10 to 100 MW(t) are still urgently needed, however. This is possible, even in areas drawing tax revenues from only a small population. In Saskatchewan, whose whole population is only equivalent to Birmingham, the Boundary Dam project uses amine scrubbing on a 100 MW(e) plant, and since opening in 2014 has sequestered over 5m t of CO2.
Approximate costs and the route to market
The future of chemical looping will be in integration with iron and/or hydrogen production, chemicals, and energy storage. Such processes will almost certainly be more expensive than elegant spreadsheet simulations suggest, and the market forces cannot be depended on in the short term, as illustrated by the closure in 2020 of the Petro Nova project, a major enhanced oil recovery project in Texas, where plunging oil prices and higher than expected CO2 sequestration costs rendered the project uneconomic.
Processes like chemical looping cannot progress fast enough without adequate funding. As we need to achieve net zero carbon emissions by 2050, progress must be faster than the current glacial pace, and this requires significant government funding and regulation. Without significant investment in technologies for a carbon constrained world by governments like that of the UK, no climate change goals will be met.
Industrialised nations with strong research bases need to show ambition. We should not rely on century-old technologies such as amine scrubbing – regardless of whether the Treasury can see short term returns. If the UK is serious about being a science superpower, leading on climate change, and bolstering the economy with new industrial ventures, a first sure step would be the creation of a chemical looping demonstration centre.
References
1. Richard K. Lyon and Jerald A. Cole, Unmixed information: an alternative to fire, Combustion and Flame, 121, 2000
2. https://cordis.europa.eu/project/id/241302/reporting
3. Anders Lyngfelt and Bo Leckner, A 1000 MWth boiler for chemical looping combustion of solid fuels – Discussion of design and costs, Applied Energy 157, 475-487, 2015
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




