A Safer Management of Ammonia
Seshu Dharmavaram discusses his research into anhydrous ammonia and how better management of it can positively affect decarbonisation goals
COMPREHENSIVE research into anhydrous ammonia releases and spills in 2022 confirmed that the use of refrigerated ammonia positively impacts its safe storage, handling, and transportation, and moves us one step closer to a net-zero future.
If hydrogen is the fuel of the future, then anhydrous ammonia is the top contender for transporting it to make it available when and where needed. Currently 80% of ammonia produced in the world is used in the fertilizer industry and only 20m t/y is globally traded. The annual production however is set to rise steeply from 180m t/y to 500m t/y or more by 2050. Why? Because ammonia’s additional applications, as a fuel for electricity production, marine transport, and as a hydrogen carrier, make it indispensable for meeting global decarbonisation and net-zero goals.
Ammonia is all around us – in the air, the soil, our bodies, our household cleaners. And soon anhydrous ammonia will be produced and transported worldwide for production of blue and green hydrogen to power communities and economies. However, at high concentrations, it is toxic and can cause severe irritation to the eyes, nose, and throat. Depending on the length of exposure and the concentration, it can also result in very serious injuries and even fatalities.
Studying and understanding the behaviour of ammonia is therefore crucial to advancing the use cases for it and one area that requires research is spillage of refrigerated ammonia. While field test data currently exists for high-pressure (ambient temperature) two-phase releases of ammonia, no field tests have ever been done for cold (refrigerated) ammonia liquid spills on dry land or onto water.
Our team conducted exactly this research – the Red Squirrel Tests – to determine the source term and dispersion characteristics for high-pressure (ambient temperature) liquified ammonia and low-pressure (cold/refrigerated) liquified ammonia in the form of two-phase releases and liquid spills. We want to help develop safer designs and processes for storage, handling, and transport of anhydrous ammonia for not just Air Products but also the wider industry that is going to find itself very busy in the coming decades.
Ammonia’s additional applications... make it indispensable for meeting global decarbonisation and net-zero goals... However, at high concentrations, it is toxic

The Red Squirrel Tests
We conducted the tests at the Det Norske Veritas (DNV) Spadeadam site in northern England where the native and rather rare red squirrels can still be found. We named our tests after these plucky animals.
The DNV research site has been used since the 1970s for conducting major hazards research but the weather conditions are variable and unpredictable. We wanted dry weather but with a bit of wind so as to avoid off-site impacts – it doesn’t help when the wind takes a plume in the opposite direction from downwind sensors. These considerations meant that it took us almost all of 2022 to conduct these tests in optimum conditions!
We performed the tests as horizontal releases through a 6 mm opening from a discharge vessel elevated 1 m from the ground. For the test with refrigerated, pressurised ammonia, we directed the release at a 45° downward angle so as to collect most of the spillage – this however made studying the transition behaviour much harder.
RS-1, RS-2 and RS-3
We conducted three sets of tests. For the refrigerated, unpressurised releases for RS-1, we wanted a large release rate over a relatively short period of time. We achieved this by simultaneously using an ammonia release vessel (ie a hopper) as well as an ammonia discharge vessel with a release orifice. This way a large amount of cold liquid was released into the concrete bund in a short time. The RS-2 and -3 tests that featured refrigerated and ambient temperature pressurised releases used only the ammonia discharge vessel.
RS-1
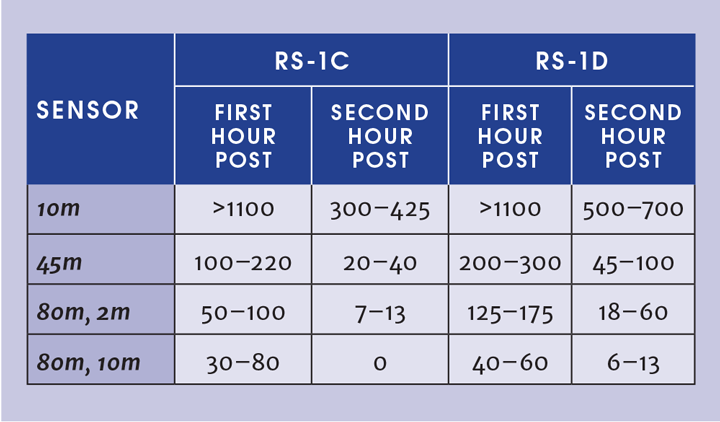
The results of RS-1 on dry concrete: We released the ammonia for approximately six minutes into the concrete bund, following which, the bund mass and field concentrations were monitored for several hours. The sudden release of cold/refrigerated ammonia into the concrete bund resulted in a pool – and we let it boil off overnight to examine the evaporation behaviour under varying weather conditions.
Initially, the vaporisation rate was rather high because the bund was at ambient temperature. The concrete base of the bund cooled almost instantly when ammonia was poured into it and this in turn slowed the vaporisation rate.
With the release, the temperature of the bund base plummeted from the ambient temperature of 18°C to about −34°C, the temperature of the refrigerated ammonia. Over the next 358 seconds of the release, the temperature continued to drop until it reached −60°C. It then warmed back up to −33°C and continued evaporating.
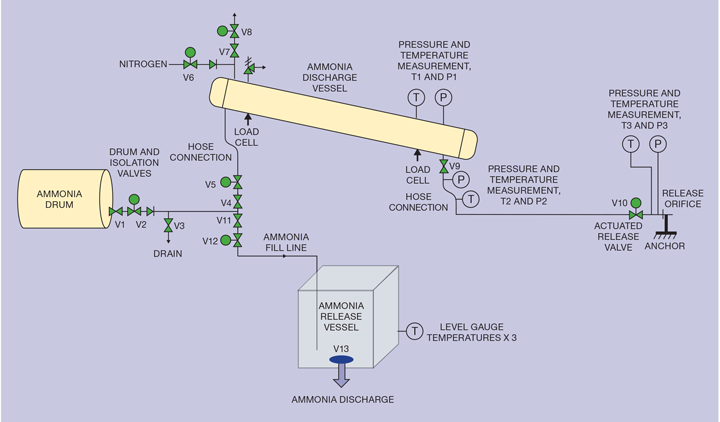
The ammonia concentrations in the air measured in the immediate vicinity of the bund were high but, when measured 45 m downwind, reduced to 300 parts per million (ppm) or less. And this reduced further over time because the drop in temperature slowed the vaporisation. Measured in the second hour after the release, the concentration had dropped to below ERPG-2 levels at 45 m.
This is critically important. Emergency Response Planning Guidelines (ERPG) published by the American Industrial Hygienists Association are the estimated concentrations at which most people will begin to experience health effects if they are exposed to a hazardous airborne chemical for one hour. Different chemicals have different ERPG concentrations depending on how toxic they are. The ERPG-2 and ERPG-3 are the upper limits for concentrations and for ammonia they are 150 and 1500 ppm, respectively. ERPG-2 is a concentration above which irreversible injuries can occur while very serious injuries and potential fatalities can occur based on exposure time at concentrations above ERPG-3.
The results of RS-1 on water: When refrigerated ammonia was released onto water, more than 50% of the ammonia was absorbed by the water and the remainder behaved like a short-duration vapour release, or “puff”. This confirms my earlier theoretical estimates1 and is a good result considering that large amounts of ammonia will be transported by ships and barges on oceans, seas, and inland waterways.
Due to the water absorbing the bulk of the ammonia, the pH of the water rose to about 11 and the resultant aqua ammonia concentration mass registered at 7%. An hour later, the concentration of ammonia in the water had decreased by 1–1.5% and the pH had decreased only slightly, which was as we expected, because the vaporisation rate of ammonia from the pool was extremely low.
The low vaporisation rate also meant that although the concentrations of ammonia in the air were initially higher for the releases into the water than for the releases onto concrete, the peak concentrations did not persist for more than a few seconds.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




