Producing ammonia with plasma

CHEMICAL engineers have produced ammonia from nitrogen and water using plasma, in a process that they say could be a step towards greener, more distributed production of this key industrial chemical.
Researchers at the Department of Chemical and Biomolecular Engineering at Case Western Reserve University, US, say that there is a growing need for a greener, scalable form of ammonia production to replace industry’s Haber-Bosch process.
Ammonia is currently produced by reacting N2 with H2 over an iron-based catalyst at high pressure (15m–30m Pa) and high temperature (400–500°C). According to figures from the IEA, ammonia production uses more energy and produces more greenhouse gas emissions than any other large-volume chemicals manufacturing process. This is because the Haber-Bosch process relies on coal and natural gas for hydrogen feedstocks and these fossil fuels are catalytically converted in multiple steps before ammonia synthesis takes place. Due to the low single-pass conversion efficiency (15%) and the high temperatures and pressures required, plants using Haber-Bosch are large and centralised to be economical, and this makes it difficult to integrate them with greener sources of H2 such as electrolysis powered by renewable energy.
The Case Western Reserve team says that while alternative photochemical and electrochemical processes are being explored, they suffer from poor selectivity related to catalysts. By using plasma in a process that requires no catalyst, the team is striving to overcome these issues while doing away with the need for hydrogen feedstocks from fossil fuels.
The team has developed a hybrid electrolytic approach that uses a plasma electrode to produce ammonia from N2 and water at ambient temperature and pressure.
“In our system, the ammonia is formed at the interface of a gas plasma and liquid water surface and forms freely in solution,” said study author Mohan Sankaran.
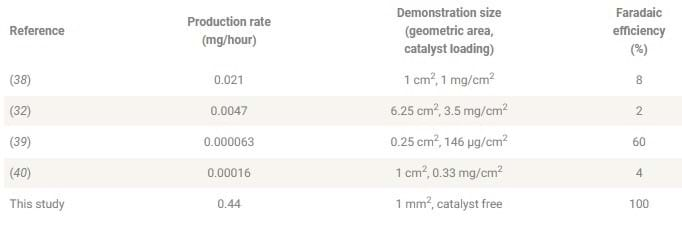
While the system demonstrates high production rates and efficiencies compared to other electrochemical methods at ambient temperature and pressure (see table), it suffers from high power requirements. It uses 2,270 kWh to produce 1 kg of ammonia compared to just 9–13 kWh for Haber-Bosch processes.
The team writes that future studies should be aimed at lowering the energy consumption by enhancing electron generation in plasmas or exploring other electrode materials, and that the continual reduction in the cost of renewable energy should help improve economics.
“Considering the overall cost of ammonia that is associated with production, capital, shipping and storage costs, our technology could be economically attractive by enabling smaller-scale distributed networks,” the authors write.
Science Advances: http://doi.org/c2tp
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




