Plasma synthesis turns CO2 and CH4 into fuels and chemicals
RESEARCHERS have reported a “major breakthrough” in reforming CO2 and CH4 into liquid fuels and chemicals, by using a highly selective one-step process at ambient conditions.
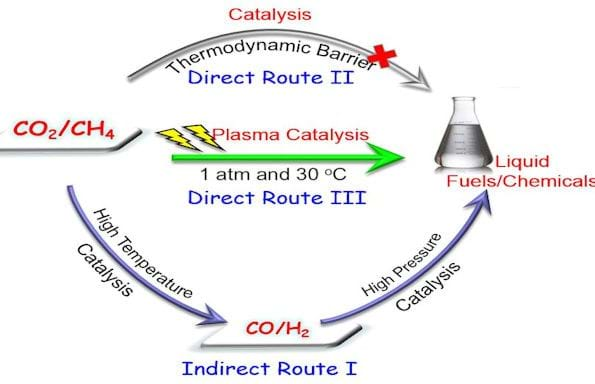
Producing value-added products such as acetic acid, methanol and ethanol from CO2 is regarded as a key element in a sustainable, low-carbon economy. However, this can be difficult in a commercial context due to the molecular stability of CO2 and CH4, which can be used as a source of hydrogen in reactions.

For example, catalytic hydrogenation requires high operating pressures, while dry reforming of methane is thermodynamically unfavourable as a one-step process, while a two-step catalytic route requires high temperatures and pressures.
But these issues were able to be bypassed by a new plasma synthesis method, which is published in Angewandte Chemie. The process, developed by a British and Chinese research team, used plasma, the fifth state of matter, for the activation of CO2 and CH4 into higher value products and formaldehyde.
This was possible at 30°C by using a novel non-thermal plasma reactor with a water electrode. Non-thermal plasmas are a state of matter that exists at temperatures as low as ambient conditions, but electrons are highly energetic. This is sufficient to activate inert CO2 and CH4, and produce a variety of chemically-reactive species from which products could be formed.
In their work, the total selectivity for liquid chemicals was approximately 50–60%, with acetic acid as the major product.
They also reported that the introduction of a catalyst into the plasma chemical process, known as plasma-catalysis, could tune the selectivity of target chemicals. This could produce formaldehyde, which could not be generated without the catalyst.
Xin Tu, from the University of Liverpool, said: “These results clearly show that non-thermal plasmas offer a promising solution to overcome the thermodynamic barrier for the direct transformation of CH4 and CO2 into a range of strategically-important platform chemicals and synthetic fuels at ambient conditions.”
“This is a major breakthrough technology that has great potential to deliver a step-change in future methane activation, CO2 conversion and utilisation and chemical energy storage, which is also of huge relevance to the energy and chemical industry and could help to tackle the challenges of global warming and greenhouse gas effect,” he added.
According to the researchers, a further benefit of using plasma is that systems have the flexibility to be scaled up and down. When compared to thermal processes, they can also be rapidly started up and shut down, reducing the energy cost.
Angewandte Chemie: http://doi.org/cdz4
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




