Novel particles for photocatalytic water treatment
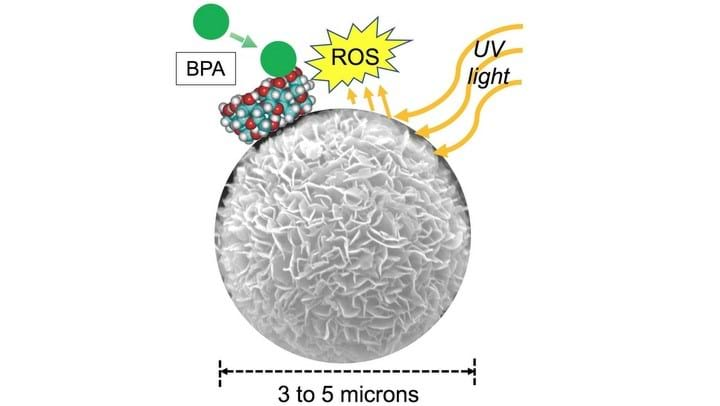
RESEARCHERS at Rice University, US, have developed novel micrometre-sized, titanium dioxide (TiO2) particles that can trap and degrade bisphenol A (BPA). Further development could lead to a novel water treatment.
BPA is a synthetic chemical used to make plastics. It is commonly used to coat the insides of food cans, bottle tops, and water supply lines. BPA was also once used as a component of baby bottles. BPA from containers can seep into food and drink, and though low-level exposure is considered safe, prolonged exposure is thought to affect the health of children and to contribute to high blood pressure.
The developed particles utilise surface attached cyclodextrin molecules – cyclic, sugar-based molecules - to trap BPA. Exposure of the particles to ultraviolet (UV) light, enables photocatalytic degradation by reactive oxygen species (ROS) produced by the titanium particles. In this case the ROS are hydroxyl radicals.
The particles are better performing than the commercially available TiO2 catalyst P25 (Evonik), exhibited by faster removal of contaminants. In addition, the developed particles can be easily recovered using microfiltration and then recharged with cyclodextrin for further use.
Danning Zhang, graduate student at Rice and lead researcher, said the reported catalyst is first generation work for demonstration purposes and that the catalyst still requires further development. “We are working on extending the surface coating lifetime,” Zhang said. The approach used to enhance TiO2 affinity and bring pollutants closer to the reactive sites could be improved. The researchers are investigating coatings more resistant to radical attack that increase the sorption capacity and photocatalytic stability of the TiO2 particles.
Titanium dioxide hierarchical spheres with surface anchored cyclodextrin
ROS are capable of degrading BPA, and they are produced when TiO2 is exposed to ultraviolet light. However, ROS degrade quickly so BPA has to close in order to enable effective attack.
The researchers created self-assembled TiO2 hierarchical spheres (TiO2-HS) from TiO2 nanosheets using a hydrothermal reaction. The particles were between 3–5 μm in size. The use of nanosheets as building blocks resulted in particles with a large surface area – three times larger than that of commercially-available Evonik P25.

Close up the spheres resemble flowers, the TiO2 arranged into collections of petal-like structures. The “petals” provide surface area onto which the researchers anchored a food-grade cyclodextrin, carboxymethyl-β-cyclodextrin, to produce CD-TiO2-HS. The molecules are attached via a chemical bond, Ti-O-C, where C is a sugar carbon.
Cyclodextrins (CD) are benign molecules, often used in food and drink. They are also two-faced molecules. They have a hydrophilic outer surface enabling aqueous dispersibility of the particles, and a hydrophobic cavity. BPA is also hydrophobic and is therefore naturally attracted to the cavity where it becomes trapped.
Exposure of the particles to UV allows the BPA to then be degraded into harmless chemicals. The researchers found that 200 mg of the spheres per litre of water was able to degrade 90% of the BPA in the water in one hour. The water contained 20ppm of BPA. The process took twice as long with TiO2-HS, particles without attached cyclodextrin.
The CD-TiO2-HS was able to remove over 99% of various contaminants with dissimilar hydrophobicity within 2 hours under a low-intensity UV input. In this case the contaminants were BPA, bisphenol S, 2-naphthol, and 2,4-dichorophenol.
Researchers found that BPA removal efficiency started to decrease after 300 hours as the ROS starts to degrade the cyclodextrin molecules. A significant loss was observed after 400 hours. Therefore, periodic replacement or replenishment of the particles would be required. This is the same for other catalysts, including TiO2 slurry.
Typically, the separation and recovery of photocatalytic TiO2 slurry requires more energy than the UV lights needed for photoexcitation.
“Most of the processes reported in the literature involve nanoparticles,” said Danning Zhang. “The size of the particles is less than 100 nanometres. Because of their very small size, they’re very difficult to recover from suspension in water.” The developed nanoparticles are bigger than reported particles. “That means we can use low-pressure microfiltration with a membrane to get these particles back for reuse,” Zhang said, adding: “It saves a lot of energy.”
In experiments the researchers found that they could recapture more than 99% of the particles from both batch and continuous flow reactors using microfiltration. The researchers were able to achieve full reactivation of the particles by re-anchoring CD to the surface, a task which would be offset by the energy savings achieved in recovery.
The particles were created in the lab of Pedro Alvarez, co-author and professor of civil and environmental engineering at Rice. He said that the developed material overcomes two technological barriers associated with photocatalytic water treatment.
He said: “First, it enhances treatment efficiency by minimising scavenging of ROS by non-target constituents in water. Here, the ROS are mainly used to destroy BPA. Second, it enables low-cost separation and reuse of the catalyst, contributing to lower treatment cost,” he said. “This is an example of how advanced materials can help convert academic hypes into feasible processes that enhance water security.”
Overall, CD-TiO2-HS particles are an attractive candidate for photocatalytic water and wastewater treatment which would enable the degradation of organic pollutants resistant to chemical decomposition.
The work carried out by the researchers at Rice fits into the technologies developed by a Rice-based research centre, the Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment. The centre’s goal is to facilitate access to clean water almost anywhere in the world. To achieve this goal, it is developing efficient modular water treatment systems that can be easily deployed, and can utilise unconventional sources to provide humanitarian water or emergency response. The centre also develops systems to treat and reuse challenging industrial wastewaters in remote locations.
Environmental Science and Technology: http://doi.org/gfgm5j
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




