Dual-polymer hydrogel can respond dynamically to its environment

A HYDROGEL has been developed that can bend, twist, or stick together when exposed to certain solutions, and could be used to create LEGO-style hydrogel blocks for microfluidics.
Hydrogels solidify when they become crosslinked, and these crosslinked polymers are held together by either covalent or ionic bonds. Covalent bonds are strong but irreversible, so that conventional hydrogels with covalent crosslinking are stable and can be turned into complex shapes, but they don’t respond to stimuli and exhibit poor adhesion. Polymers with ionic bonds are weaker and the bonds can be reversed, leading to hydrogels that exhibit adhesion and have some flexibility, but lack structural integrity.
Now, researchers at Brown University, US, have created a dual polymer hydrogel with both covalently- and ionically-bonded polymers that can be shaped using light-directed 3D printing. "Essentially, the one polymer provides structural integrity, while the other enables these dynamic behaviours like bending or self-adhesion," said Thomas Valentin, lead author of the study. "So putting the two together makes a material that's greater than the sum of its parts."
The new material has one covalently-crosslinked polymer, poly(ethylene glycol) diacrylate (PEGDA), and one ionically-crosslinked polymer, poly(acrylic acid) (PAA). The researchers created a “soft gripper” that acts like a pair of tweezers to pick up an object. The material has PEDGA on one side and a PEGDA-PAA mixture on the other. The PEGDA-PAA hydrogel immersed in deionised water will swell, but contracts when immersed in high concentrations of cations. If a volume change occurs in one material, the gripper will bend to accommodate the stress, thereby pulling two pieces together that can hold objects weighing around a gramme.
"There's a lot of interest in materials that can change their shapes and automatically adapt to different environments," said Ian Wong, Assistant Professor of Engineering at Brown. "So here we demonstrate a material that can flex and reconfigure itself in response to an external stimulus."
Different geometries of PEGDA and PEGDA-PAA hydrogels can result in complex shape changes with bending and twisting.
When exposed to a moderate cation concentration, the hydrogel exhibits self-adhesion rather than contraction, meaning that if the hydrogel is severed, it will bond back together when placed in the cation solution. As an example, the team 3D-printed a hydrogel salamander, cut off the tail, and then reattached it within the cation solution.
Hydrogels are generally nontoxic, which makes them suitable for use in microfluidic devices for biomedical testing. However, it is difficult to create the complex channels needed for microfluidics in conventional hydrogels. By using 3D printing, the researchers can incorporate complex microfluidic channels into LEGO-shaped hydrogel blocks. The blocks can then be assembled and sealed together with self-adhesion using the cation solution.
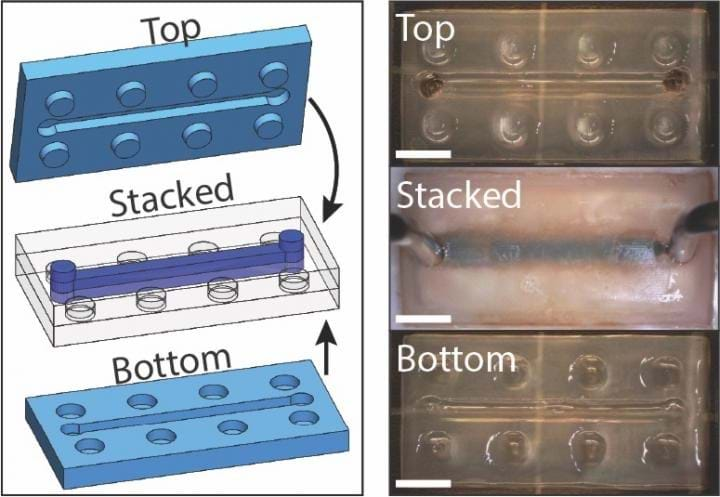
"The modular LEGO blocks are interesting in that we could create a prefabricated toolbox for microfluidic devices," said Valentin. "You keep a variety of pre-set parts with different microfluidic architectures on hand, and then you just grab the ones you need to make your custom microfluidic circuit. Then you heal them together and it's ready to go."
Polymer Chemistry http://doi.org/c4mg
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




