Covid-19 antibody test gives results in 20 minutes
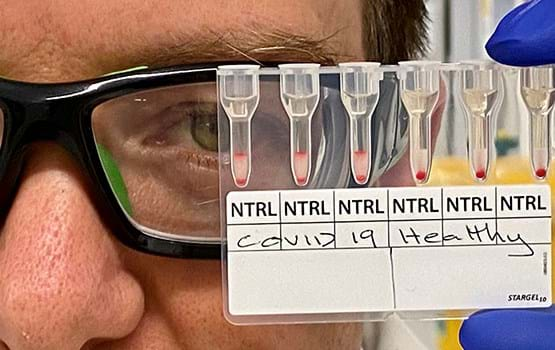
A BLOOD test has been developed by researchers in Australia which can detect Covid-19 antibodies in 20 minutes. The test is easily scalable and up to 700 tests per hour could be performed using hospital equipment.
The research was led by Bioresource Processing Institute of Australia (BioPRIA) and the Chemical Engineering Department at Monash University, Australia. The researchers developed a method to detect antibodies caused by Covid-19 using an agglutination assay, which determines the presence and amount of a substance in blood samples. The positive cases cause an easily identifiable agglutination or a clustering of red blood cells, and negative samples don’t cause any clustering. Recent cases could be identified using 25 µL of blood plasma.
The test can be used to determine if someone recently had the infection, giving it the potential to be used as part of vaccine trials to determine if antibodies have increased in response to a vaccine candidate. It can also be used for population screening and contact tracing, and to determine for how long antibodies persist after infection. Up to 200 samples per hour could be tested in a lab setting, rising to 700 per hour using high-grade diagnostic machines in hospitals.
Simon Corrie, Senior Lecturer in Chemical Engineering at Monash University, said: “Detection of antibodies in patient plasma or serum involves pipetting a mixture of reagent red blood cells (RRBCs) and antibody-containing serum/plasma onto a gel card containing separation media, incubating the card for 5–15 minutes, and using a centrifuge to separate agglutinated cells from free cells. This simple assay, based on commonly-used blood typing infrastructure and already manufactured at scale, can be rolled out rapidly across Australia and beyond. This test can be used in any lab that has blood-typing infrastructure, which is extremely common across the world.”
Banaszak Holl, Head of Chemical Engineering at Monash University, said: “This simple, rapid, and easily scalable approach has immediate application in SARS-CoV-2 serological testing, and is a useful platform for assay development beyond the Covid-19 pandemic. We are indebted to the work of our PhD students in bringing this to life. Funding is required in order to perform full clinical evaluation across many samples and sites. With commercial support, we can begin to manufacture and roll out this assay to the communities that need it. This can take as little as six months depending on the support we receive.”
ACS Sensors http://doi.org/gg5f4t
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




