Liquid Metal: a Catalyst for Change?
Requiring temperatures into the hundreds of degrees to work, solid catalysts prove incredibly energy intensive. Kerry Hebden spoke to researchers at the University of Sydney who have developed a liquid metal alternative that could make the chemical industry greener
FOR CENTURIES, catalysts have been used to make a variety of products, from beer in the 1500s, to plastics, fertilisers, and fuels in recent years. Typically, most modern-day industrial chemical processes use solid catalysts to activate reactions.
Despite the common use of solid catalysts, their efficiency has been questioned. Catalytic converters in cars, for instance, typically use platinum or palladium deposited as thin layers onto a ceramic honeycomb to maximise the surface area and to keep the amount of metal used to a minimum. These metal atoms are therefore fixed in place as they are bonded to a solid support. Does this lack of mobility limit their performance as a catalyst? At least one team thinks so.
What is a catalyst?
A catalyst is a substance that makes chemical reactions occur faster and more easily without itself being consumed. Even as early as the 19th century, it was found that a chemical reaction between two gaseous reactants can occur on a metal surface without the metal being chemically changed. Humphrey Davy is often credited with the discovery, and he frequently used platinum and palladium in his experiments.
These two metals, along with nickel, are still widely used as catalysts today. That’s because their surfaces adsorb reactant molecules strongly enough so that reactions can take place, but not so strongly that the product molecules can’t break away.
Kourosh Kalantar-Zadeh, head of the University of Sydney’s School of Chemical and Biomolecular Engineering, had a hunch that a surface of liquid metal, when used as a catalyst and reaction media, would be more active than a solid metal one, and therefore aid in more efficient reactions. “Liquid metals can offer some things that solids cannot,” Kalantar-Zadeh says. “For example, they have a lower entropy than solid materials. Metallic atoms can freely move in liquid media, and liquid metals can have more intense localised vibration, enabling them to make or break atomic bonds easier than solids.”
Together with Junma Tang, and Arifur Rahim, who are also based at The University of Sydney, the team set about testing the idea by dissolving tin and nickel in another metal – a gallium-based liquid metal – so that the metallic tin and nickel atoms could remain dynamic. Gallium was chosen because it is liquid near room temperature (it has a melting point of around 29.8°C), is non-hazardous, and is known to dissolve other metal solutes in varying concentration. As a proof-of-concept, decane, an alkane hydrocarbon used in the production of engine fuels and adhesives, was used as the feedstock. It was heated to around 150°C and added to the catalyst.
During their experiments, the team found that the nickel and tin stayed dissolved, which allowed the atoms to migrate to near the surface of the gallium and catalyse. This altered the hydrocarbons to make propylene, a high-energy fuel crucial to many industries. “By remaining dynamic, these metals effectively turned into ‘super’ catalysts, as they allowed reactions to take place at a lowered temperature, and with less energy, which results in increased efficiency,” Kalantar-Zadeh says.
The team’s next step was to try different concentrations of nickel and tin, and different metals to see if this helped increase the product output. But key to their idea of developing greener catalysts was being able to use them with a renewable hydrocarbon. The team chose canola oil as they realised that this too can be converted into propylene.
To test the synthesis of propylene from canola oil, the team scaled up their experiments by building a simple reactor.1 Inside, metallic meshes coated with a thin layer of gallium, nickel, and tin were stacked together to increase the surface-to-volume ratio of the liquid catalysts.
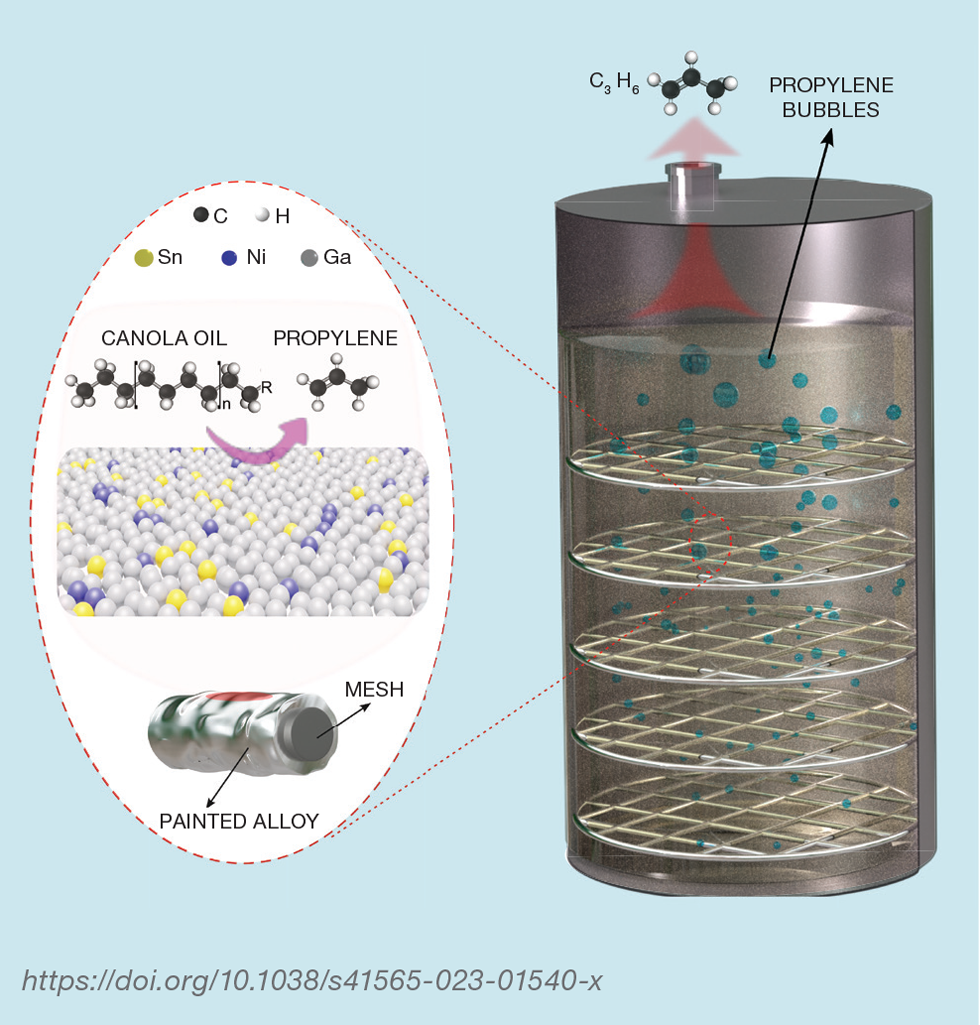
The reaction system was heated and maintained at a temperature of around 150°C, and argon gas was purged through the system to recover the produced gaseous species. The efficiency of propylene production was measured by a flow meter combined with gas chromatography analysis. The tests revealed that a high selectivity of around 94.5% of propylene was obtained by using 2 g of the gallium/nickel/tin catalyst. A continuous reaction of over 720 h was also achieved.
The group has also been involved in experiments with platinum dissolved in gallium.2 Dissolved platinum can drive catalytic reactions at lower temperatures (45°C to 70°C), and when used for electrochemical methanol oxidation, the platinum atoms exhibited an activity several orders of magnitude higher than existing solid platinum catalysts of the same weight.
The experiments proved so successful that the team plans to do more, and with different metal varieties and quantities. “In the long-term, our plan is to apply for a Centre of Excellence (CoE), and bring on board other institutions, and some recognised companies, with an aim of discovering all unknowns about liquid metal reaction media, and eventually going commercial,” Kalantar-Zadeh says. Funded by the Australian Research Council, such CoEs are key forums for researcher-driven, transformational, and collaborative research between universities and other organisations, both in Australia and overseas.
And the team don’t want to stop at producing propylene. Just about any chemical that can be produced could be made greener with liquid metal catalysts, Kalantar-Zadeh says. “For instance, companies have been making ammonia (NH3) via the Haber-Bosch process for over 100 years. But it is intrinsically inefficient and requires hydrogen and nitrogen reacted together at specific temperatures, and high pressures. We could change that.”
I am very optimistic that with liquid metal catalysts we can find solutions for one chemical after another and make reactions more efficient so that we can reduce our impact on the environment
The team are also testing mixing different metals together so that multiple products could be produced from the same reactor. “Currently, the chemical industry generates more than 10% of global greenhouse emissions annually, and the number is rising,” Kalantar-Zadeh says. “I am very optimistic that with liquid metal catalysts we can find solutions for one chemical after another and make reactions more efficient so that we can reduce our impact on the environment.”
References
1. https://www.nature.com/articles/s41565-023-01540-x
2. https://www.nature.com/articles/s41557-022-00965-6
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




