Friendly Foams
Building the UK’s first captured-carbon polymerisation plant
HUMANS have become alarmingly proficient at transferring carbon out of the ground and into the air. As a result, chemical engineers face many challenges in guiding industry away from fossil fuels and delivering economical carbon capture and utilisation (CCU) technologies.
This challenge brought me to Econic Technologies in the UK – a catalyst technology company currently focussed on commercialising its CO2-to-polymer technology. The proprietary catalyst technology, which can be retrofitted, involves using CO2 to partly-replace oil derivatives as feedstock to make more environmentally-friendly foams and other polymers.
A wide scope of applications could be made more environmentally-friendly: captured carbon dioxide can be converted into building materials (such as insulation foam, coatings and adhesives); furniture components (such as the flexible foams in beds and sofas); materials for the automotive industry; and high-performance foams used in anything from surfboards to running-shoe soles.
My role at Econic was to lead the polyol demonstration plant project, the UK’s first captured-carbon utilisation plant. The project focus was not on CO2 capture from industry, but utilisation of this captured CO2 as a feedstock in a chemical reactor. The plant will be used to demonstrate the potential of the novel technology to potential customers.
For every tonne of CO₂ used in the manufacture of polyols, studies have shown that the partial displacement of oil-based feedstock results in a reduction of CO₂ emissions by a further 2 tonnes
The environmental benefits of using CO2 in the production of polyols, or indeed other polymeric variants, is two-fold. There is the advantage of turning this waste product from an environmental burden to a useful raw material with the potential to generate profit. And for every tonne of CO2 used in the manufacture of polyols, independent studies1,2 have shown that the partial displacement of oil-based feedstock results in a reduction of CO2 emissions by a further 2 tonnes. In other words, by not making the propylene oxide that has been replaced by using 1 t CO2, we avoid the creation of a further 2 t of CO2 emissions.
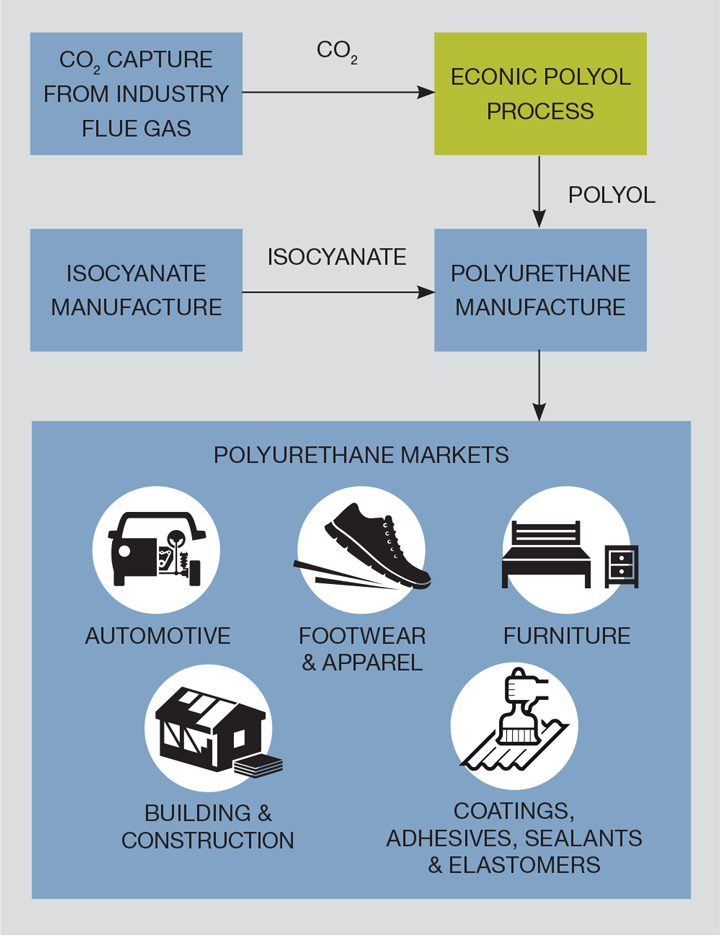
How it works
The reaction grows a polymer from a starter molecule by adding both carbon dioxide and another typical industry feedstock, propylene oxide, as monomer units. These polymers are called polyether carbonate polyols, which is the final product in the demo plant. These polyols are typically ‘foamed’ with isocyanates at polyurethane plants to generate bulk foam slabs, or further processed for other applications.
The physical properties of polyurethane can be optimised for each application by tweaking the polyol’s length (molecular weight), CO2 content, and functionality.
Econic is a catalyst company, not a bulk polymer producer, and we are working to get our technology into the process plants of the world’s largest polyol producers. With this objective, our research team had a major breakthrough in the form of a tunable catalyst system. This tunable system enables the level of CO2 incorporated into a polyol to be specified, allowing optimisation for a specific application or end-product.
With the tunable catalyst system, the producer can tailor the polyol properties by varying the amount of propylene oxide replaced with CO2, offering greater potential to broaden properties and applications
Current technology manufactures polyols using oil-derived epoxides as raw materials. The most common feedstocks are propylene oxide and ethylene oxide, which are produced by oxidation of propylene and ethylene respectively. Econic’s alternating catalyst system (see Figure 2) maximises the amount of the carbon dioxide in the polyol, by replacing every other propylene oxide molecule in the polymer chain with CO2. This typically results in a relatively stiff polyol, which can be used to make polyurethanes for a range of applications, but may be considered too stiff for some unless it can be blended with conventional polyols.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




