Hydrogen in Vehicular Transport

GOOGLE the subject of this article, and you'll get hundreds of thousands of results. It is therefore a challenge to cover everything in less than 2,500 words!
So, in this article a number of vehicular transport types will be considered, albeit not at an exhaustive level; some of the challenges facing each will be described, and a number of potential opportunities will be noted.
In 1794 Robert Street patented what is arguably the internal combustion engine concept, which used a liquid fuel (petroleum), and then followed it by building a working engine. And for the last 225 years his invention has been the backbone of vehicular transport – to the point at which around 16% of the entire world population now owns a car1.
The big decision is, are we going to replace (carbon-emitting) petrol in an internal combustion (IC) engine with (carbon-free tailpipe emissions) hydrogen, or are we going to use the hydrogen differently, such as in a fuel cell? Or is the answer somewhere in between as a hybrid? This is probably a good place to start.
Hydrogen internal combustion engines
The advantage of using IC engines such as Wankel and piston engines burning hydrogen instead of petrol, is that the cost of retooling for production and fitting the prime mover into existing body shell and floor pan designs is much lower.
The optimum fuel-to-air ratio for petrol is 14.7 (14.5 for diesel): for hydrogen it is 29. However, to minimise the formation of NOx, typically hydrogen engines are designed to use about twice as much air as this. Unfortunately, this also reduces the power output to about half that of a petrol engine of the same physical size, so to make up for this, hydrogen IC engines are usually much larger than petrol engines.
Other issues also need addressing, such as the increased potential for pre-ignition (knock or pinging) because hydrogen flame speeds are higher than those for petrol or diesel fuel.
A number of cars have been produced with hydrogen-fuelled IC engines: BMW produced the Hydrogen 7 in 2005–2007, and Mazda produced a hydrogen version of its rotary-engine RX-8, for instance. Neither of these are in current production.
There is, however, a niche market for hydrogen IC vehicles operating where there could otherwise be an accumulation of CO-rich fumes, such as fork-lift trucks in warehouses.
Hydrogen fuel cell cars
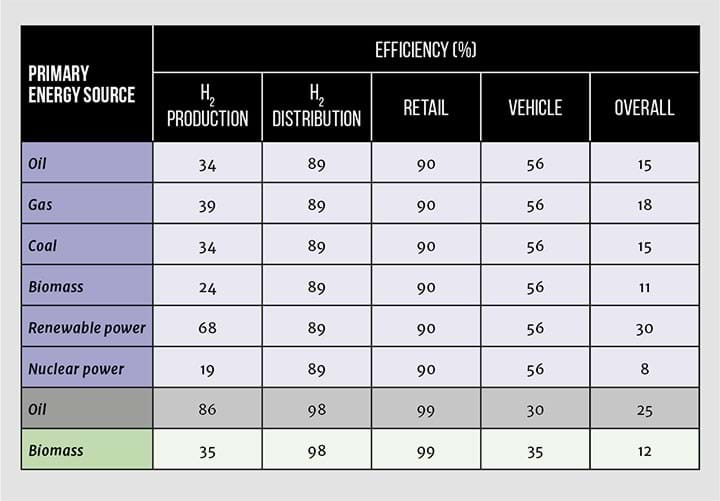
Earlier articles in this series present a case study, “Enabling Hydrogen Fuel Cell Electric Vehicles”, and look at the Toyota Mirai. Fuel cells represent a better utilisation of hydrogen energy in cars than a hydrogen-fuelled IC engine, which is only about 40% compared to the possible 56% shown in Table 12 (blue rows show hydrogen fuel cell, and grey and green show petrol and biodeisel IC). However, because of the high energy requirement to pressurise the hydrogen, the overall well-to-wheel efficiency is not impressive. And neither is the price, around twice that of the equivalent IC-engined vehicle. What is impressive, is the emissions performance, with only water vapour appearing at the tailpipe.
Honda, Hyundai, Mercedes-Benz and Toyota have production models of fuel cell cars, with scores of other manufacturers offering concept cars.
When designing a hydrogen vehicle, there is a balance to be struck between making the fuel cell tolerant to impurities in the hydrogen and requiring it to be “4 9s” grade (ie >99.99% pure). The current market is to place the onus on the supplier, avoiding additional fuel cell costs, but this, in turn has its own suite of problems (see Hydrogen supply infrastructure later).
Hydrogen fuel cell buses
In 1998 in Chicago, US and Vancouver, Canada, there was an early demonstration of fuel cell buses, but it was 2006 when they began operating in Beijing on an experimental basis. In 2004, London experimented with single-deck hydrogen-powered buses and by the end of 2011 there were eight. They are now a regular feature of London traffic.
Later this year, 20 new Wrightbus double-deck buses will be introduced into service in London. The StreetDeck FCEV uses a Ballard fuel cell, a Siemens drivetrain and a 48 kW traction battery pack. The system delivers a 322 km operating range, and a 426 km extended storage option is also available. Refuelling the bus takes approximately seven minutes3. With regenerative braking, frequent stop/start operation, a city centre route base, and its ability to accelerate rapidly into moving traffic, this promises to be an important contribution to mass transport in the approaching low-carbon era.

Other hydrogen road vehicles
Finding room for hydrogen storage tanks may be difficult in a compact vehicle like a car (see below), but vans, and trucks have a great deal more potential. Their stop/start duty – especially if a significant amount of time is spent queueing in heavy traffic – lends itself particularly well to fuel cell operation, coupled with battery storage to allow the fuel cells to generate at a fixed rate (ie base load).
Like buses, vans and trucks may also have the advantage that, parked overnight in a depot, they could be filled with hydrogen from a bulk supply, where all of the necessary safety precautions can be put in place and secured.
Hydrogen trains
In September 2018 that the first Coradia iLint hydrogen fuel cell train entered into commercial service in Germany. Now, two of the trains built by the French train maker Alstom are now operating on a 100 km stretch of line in northern Germany. Electrical energy is generated on-board in a fuel cell and intermediately stored in batteries, as described above. The battery stores energy from the fuel cell when it is not needed for traction, or from regenerated energy of the train during (electrical) braking. The batteries also allow additional support to boost energy delivery to the motors during acceleration phases.
Both the fuel cells and the hydrogen tanks are mounted on the roof, with cooler air providing additional cooling as the train moves along the tracks at up to 80 km/h.
Hydrogen trains are expected to come into service in the UK in 2022. These are not the same as the iLint models because there is insufficient clearance in some UK tunnels to permit the roof installation of tanks and fuel cells, thus the designs tend to look more ‘conventional’.
Hydrogen supply infrastructure
Storing hydrogen on-board light vehicles for use in fuel cells is one of the current limiting factors to their widespread introduction to the marketplace. This is because, as the least dense element in the universe, the chemical energy it contains per unit volume is several orders of magnitude lower than conventional hydrocarbon fuels. For example, at 0°C and 100 kPa, hydrogen has a lower heating value (LHV) of 10.8 kJ/L, whereas petrol at 15°C and 100 kPa boasts an LHV of 32,000 kJ/L. On a mass basis, this result is reversed, with hydrogen demonstrating its promise as a future energy carrier with an LHV of 120,000 kJ/kg vs petrol at 43,400 kJ/kg. Therefore, while significantly less mass of hydrogen fuel would be required to power a vehicle for the desired range, the problem arises with the volumetric storage of such a quantity.
Experience with existing prototypes reveals that a hydrogen fuel-cell car has a fuel economy of 199 km/kg. To have a typical range of 600 km, the tank of a hydrogen car needs to hold about 3 kg of hydrogen. The problems of storing hydrogen have been described elsewhere in this series, and 3 kg of hydrogen would occupy 0.076 m³ were it compressed to 70MPa, which, although an engineering challenge in its own right, is typical. But how does one get the hydrogen to the filling point?
This has long been the “chicken-and-egg” conundrum facing hydrogen for vehicular transport. There are three main options:
- bulk delivery (by road in a tube trailer);
- bulk delivery (by pipeline); and
- on-site manufacture (eg by electrolysis).
All three have obvious downsides. A large hydrogen tube trailer can carry about 900 kg at 30 MPa, or about 300 tank-fulls (neglecting additional compression loads) which is enough for 120,000 vehicle-miles. A 44 t petrol tanker holds 38,000 L. At an average consumption of 5.5 L/100 km, this equates to 161,680 vehicle miles, 35% more and faster unloading times.
Bulk delivery by pipeline would require on-site purification (eg removal of oderant and any other impurities necessary to restore it to 4 9s), and compression from street-level pressures (less than 7barg) to tank pressure, which could be 70 MPa.
On-site electrolysis and compression represents a huge energy penalty, and also has the possibility that the electrical supply infrastructure may require reinforcement, not just at the local (400 V) level but beyond into the 11 kV system.
On-board storage of hydrogen – the problem
Optimising the compromise between gravimetric (per unit mass, often expressed as kgH2/kg or wt%) and volumetric (per unit volume, often expressed as kgH2/L) storage capacities is therefore key. This naturally leads to the question: Which is more important to a vehicle, mass or volume?
To tackle this, first consider what we mean by gravimetric storage capacity: the mass of hydrogen stored divided by the total mass of the storage system and hydrogen together. The inclusion of the entirety of the storage system mass is what largely contributes to the unfeasibility of many solutions, since tanks, pipework and instrumentation equipment is heavy, compared to the mass of gas stored. The volumetric storage capacity divides by the volume of storage space required to contain the desired mass of hydrogen. Working with a set volume (once an acceptable mass of stored hydrogen has been established) allows for some unconventional and often innovative solutions, such as hydrogen buses whose storage tanks are externally located on the roof, or in some cars where tanks can be incorporated into the floor and in otherwise dead space beneath seats etc4. An obvious (but important) principle to note is that volume can be distributed around a vehicle whereas mass is mass wherever you put it. Heavier vehicles require more fuel to travel the same distance as lighter vehicles, requiring more fuel, which leads to heaver vehicles… and the cycle continues. Since the vehicle mass is likely to be the limiting factor in hydrogen vehicle design, it is often concluded that prioritising a high gravimetric capacity is the more sensible option for cars.
On-board storage of hydrogen – some solutions
Conventionally, as stated above, the solution to this problem has been to utilise extreme high pressures (up to 70 MPa) to compress hydrogen gas to such densities that enough fuel can be stored to facilitate driving ranges comparable to existing hydrocarbon fuelled vehicles. However, there are some drawbacks to this approach. For instance, the composite tanks required to safely operate at such elevated pressures are expensive to fabricate and can deteriorate structurally with repeated pressurisation unless mitigation procedures are implemented. This concern has led to the tight regulation of pressure vessel specification, manufacture and transport, resulting in a well-established, safe storage method for hydrogen5.
Although storing hydrogen as a compressed gas is a well-established technology, the development of alternative storage systems is ongoing, with a plethora of adsorptive solutions undergoing research. Adsorption is the process by which molecules adhere to the surface of a solid phase adsorbent via van der Waals intermolecular forces. This can facilitate higher storage capacities at reduced pressures but, in this application, is limited by small size and neutrality of the hydrogen molecule, resulting in very weak interactions with any solid framework. Novel materials such as covalent-organic frameworks show some promise in this area when doped with metal ions to enhance the interaction energy of the two phases6, potentially paving the way for a future where hydrogen cars are the dominant means of personal transport.
Footnotes
- Green Car reports, 29 July 29 2014 states 1.2bn cars in the world. World population estimated as 7.53bn.
- From “Towards a smart energy network: The roles of fuel/electrolysis cells and technological perspectives”, X Zhang, SH Chan, HK Ho et al, International Journal of Hydrogen Energy, March 2015.
- Electric and Hybrid Vehicle Technology International, October 2018
- Mercedes-Benz GLC F-CELL, Daimler
- Hydrogen Storage Technology, Lennie Klebanoff
- Ideal metal-decorated three-dimensional covalent organic frameworks for reversible hydrogen storage, Choi et al, https://doi.org/10.1063/1.2912525
This is the 14th article in a series discussing the challenges and opportunities of the hydrogen economy, developed in partnership with IChemE’s Clean Energy Special Interest Group. To read more from the series online, visit the series hub.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




