Fritz Haber and Carl Bosch – Feed the World
Claudia Flavell-While profiles the world-changing efforts of Fritz Haber and Carl Bosch

No chemical engineering feat better illustrates the double-edged nature of many inventions than the Haber-Bosch process. Developed by industrial chemist Fritz Haber and scaled up by the chemical engineer Carl Bosch, the Haber-Bosch process takes nitrogen from the air and converts it to ammonia.
This made it possible for the first time to produce synthetic fertilisers and produce sufficient food for the Earth’s growing population. Indeed, without the Haber-Bosch process we would only be able to produce around two-thirds the amount of food we do today, and the Earth’s population would have to shrink accordingly.
On the flipside, nitrogen is also a key raw material in the production of high explosives. When Germany exhausted its supplies of natural ammonia in the early stages of World War I, synthetic ammonia was there to fill the gap. Without the Haber-Bosch process, World War I would have been considerably shorter.
Haber, a consummate patriot, would later gain notoriety through his work on using chemicals to Germany’s advantage in World War I, and he personally oversaw and directed the first large-scale release of chlorine gas in Ypres in 1915.
Averting famine
Nevertheless, it’s the Haber-Bosch process and its contribution to feeding the world’s growing population that he is best remembered for, and rightly so. With the advent of the industrial age and the great move to the cities, fertilisers were essential for topping up soil with nutrients. Natural fertilisers such as Chilean guano were a limited resource – if science didn’t come to the rescue, famine was certain to follow.
Solving the problem earned Haber and Bosch two Nobel Prizes in chemistry: Haber in 1918, Bosch in 1931.
The problem with nitrogen is that, while it is abundant in the atmosphere, its triple bonds make the nitrogen molecule incredibly stable and therefore hard to fix. Haber was one of a group of chemists that also included Walther Nernst and Henry Le Chatelier, which had decided to tackle the problem.
Without the Haber-Bosch process we would only be able to produce around two-thirds the amount of food we do today
Pathways and blind alleys
Haber initially attempted to produce nitric oxide with the help of electric discharges, mimicking natural processes during a thunderstorm. But the yield was so low and the process so onerous that Haber dismissed it as impractical.
Haber next investigated high-temperature synthesis, with some success, and even succeeded in producing a small amount of nitrogen in 1905. But he was disappointed with the 5% yield, at temperatures of around 1000ºC. Better catalysts or higher pressure were needed, but high-pressure synthesis was in its infancy and suitable equipment scarce. Indeed, Le Chatelier, who was the first to suggest fixing nitrogen under high pressure, gave up after a particularly hefty laboratory explosion.
It was not until 1908 that Haber, working with his student Robert le Rossignol, decided to tackle the high-pressure route. It was a good choice. One year on, they patented a process that yielded some 15% ammonia, operating at a pressure of around 175 atmospheres at 550ºC over an osmium and uranium catalyst.

From lab to industry
The process was soon assigned to Badische Anilin und Soda Fabrik (better known today by its abbreviation, BASF), which tasked chemical engineer Carl Bosch with scaling up the process. Bosch later said: “It was obvious that there were three main problems which had necessarily to be settled before the construction of a plant could be undertaken. These were supply of raw materials, ie of the gases hydrogen and nitrogen, at a lower price than hitherto possible; the manufacture of effective and stable catalysts; and lastly the construction of the apparatus.”
Cheap and cheerful
Haber’s process of producing hydrogen via electrolysis did not lend itself to scale-up. Neither did any of the other known hydrogen production processes, which were either too expensive or produced hydrogen with too many impurities.
Bosch and his team eventually settled on water-gas – a synthesis gas consisting of hydrogen and carbon monoxide – as the only practical solution. The company extracted pure hydrogen using the recently-developed Linde-Frank-Caro process, which cools water gas in several steps to -205ºC, at which point all elements apart from hydrogen liquefy.
The second major contribution was substituting the uranium osmium catalyst with a more practical alternative. Osmium was unsuitable for scale up because global supplies amounted to only a few kilogrammes, and uranium was expensive and very sensitive to water and oxygen. It took Bosch’s assistant, chemist Alwin Mittasch, some 20,000 experiments to perfect a mixed catalyst based on iron oxide. It was the first of its kind, and performed as well as osmium and uranium, and was readily available and cheap. The catalyst is still in use today, and Mittasch is remembered as one of the great pioneers of catalytic chemistry.
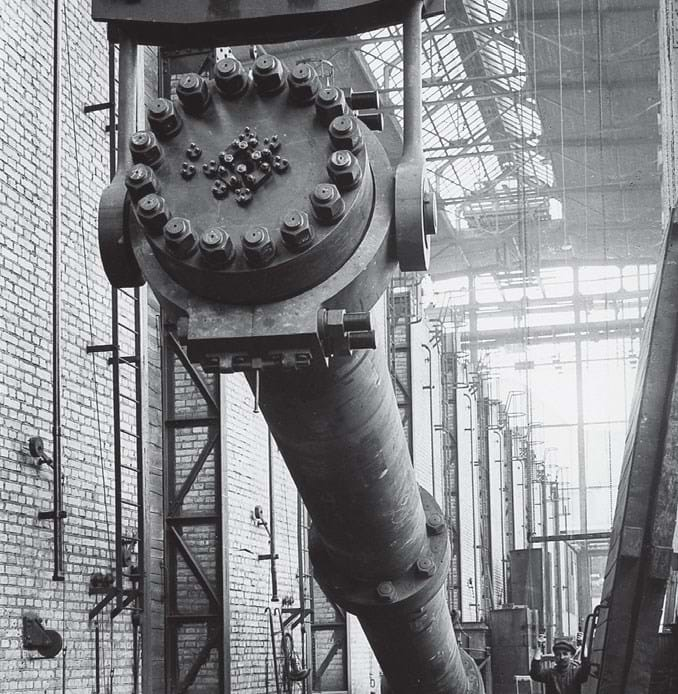
Pressure vessels
Bosch believes his greatest feat was solving the third problem, how to build a reactor that would withstand both the high temperatures and high pressures of the reaction. High-pressure chemistry was still a very new field, and suitable equipment was in short supply. The only existing high-pressure process was Linde’s air liquefaction process, a low-temperature process that used a soft soldered copper reactor which was wholly unsuitable for high temperature applications.
Bosch’s first task was to devise a new laboratory reactor, for which he re-modelled Haber’s original design into a robust, reliable reactor. Some 24 examples ran around the clock for several years while Mittasch searched for the perfect catalyst.
The small reaction chambers had none of the problems Bosch was to encounter in scale-up: the outer pressure-bearing parts were small enough that air cooling was sufficient to keep them stable, and there was only slight mechanical stress on the inner parts.
“That state of affairs was soon to change when we started to build a smaller converter as a production apparatus,” Bosch said. When Haber had made a similar attempt, the device failed after only a couple of hours of operation, so Bosch’s team designed a very sturdy, externally-heated contact tube as a reaction chamber. Even so, they took no chances: “Being cautious we had housed it in a strong, reinforced concrete chamber far away from all the busy centres of activity since in the meantime we had also become familiar with the danger of fires and flarebacks which occur, frequently with spontaneous ignition, when hydrogen emerges at high pressure,” he said.
It was a wise precaution: after 80 hours of service, the material became brittle and the tubes burst. It turned out that the hydrogen had decarbonised the perlite in the carbon steel and formed a brittle alloy with the iron.
Bosch solved the problem by designing the first lined reaction chamber – a pressure-bearing steel jacket thinly lined with a soft steel. Hydrogen was able to diffuse through the lining and was allowed to escape through grooves and holes in the jacket, to prevent a dangerous pressure build-up.
“The solution appeared simple and was in fact so but yet the entire development of the process depended on it to a greater or lesser extent,” Bosch said.
Safety considerations
Not that this was the last of Bosch’s problems: the heat exchanger was too inefficient, the pressure-bearing steel jackets still tended to buckle and explode after prolonged operation – a problem only overcome when Bosch, in a break with the convention of the times, tried heating the reactor from the inside – and compressors that were unreliable and tended to leak. This poses an unacceptable risk when the gas leaking out is hydrogen; not to mention that no chemical plant can work with compressors that fail at least once a day.
Bosch’s team not only built its own compressors, it also had to design and produce its own monitoring instruments to measure temperature, the intensity of the gas stream and the composition of the gas in the reaction chamber – off-the-shelf devices 20 years on, but unheard of when Bosch scaled up Haber’s device.
Bosch was also very conscious of health and safety, and designed numerous quick-acting safety valves and other equipment so that the plant could be shut down and evacuated with record speed. “Over the years we have been able to cull sufficient experience, especially during the war when the Oppau works were bombed night after night, and we are still of the opinion today that one cannot be too careful,” he said.
Nature knows best
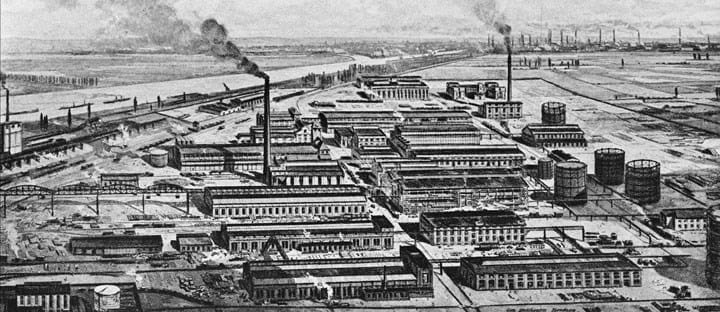
The first plant to use the Haber-Bosch process at industrial scale started up at BASF Oppau in 1913. Nearly 100 years on nothing much has changed, and the process is still used around the world.
However, Haber predicted that this would change: “Nitrogen bacteria teach us that Nature, with her sophisticated forms of the chemistry of living matter, still understands and utilises methods which we do not as yet know how to imitate. Let it suffice that in the meantime improved nitrogen fertilisation of the soil brings new nutritive riches to mankind and that the chemical industry comes to the aid of the farmer who, in the good earth, changes stones into bread.”
Originally published in March 2010
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




