Distillation Improvement Opportunities Part 6: Safety Implications from New Technologies
Roger Stokes and Michael Moosemiller outline the safety issues associated with conventional distillation towers and consider how newer technologies might behave differently with respect to safety
SEVERAL articles recently published in TCE have introduced relatively novel distillation concepts. At first glance, there are no obvious reasons why these technologies should materially alter the safety profile of a chemical operation. However, as with all new technologies, operating experience is relatively limited. There have been cases where problems have occurred and it is reasonable to speculate on how the emerging methods might perform differently to traditional distillation in a safety context.
Based on our backgrounds, first as design and operations engineers, and later through interactions with clients as consultants, we have developed a list of some of the hazards associated with “traditional” distillation (Table 1) and will look at each of these in more detail.
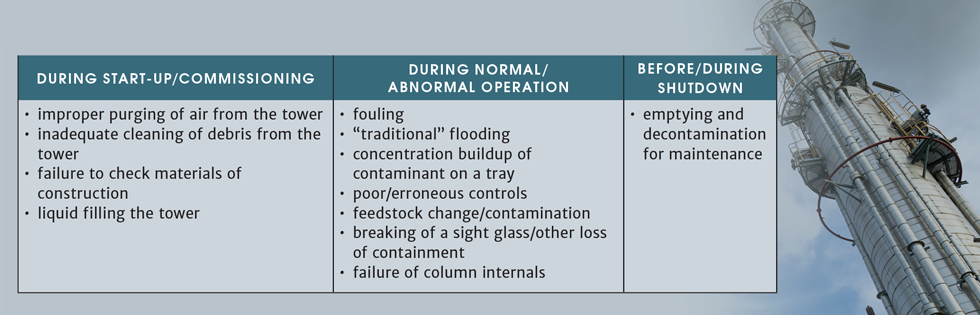
Start-up/Commissioning
Some of the biggest hazards in the life of a distillation column present themselves during the initial commissioning phases, or recommissioning after a turnaround.
Improper purging of air from the tower
During installation, or after a turnaround, a distillation tower will initially be full of air. The air needs to be removed before introducing hydrocarbons or other flammables. A common way of doing this with readily available utilities is to: (a) push out the air by opening steam to the tower base and venting from the top, then (b) replace the steam with fuel gas or similar. Step (a) is usually considered to be complete when steam can be seen venting from the top of the tower (this takes some hours, since much of the steam initially condenses as a result of heating the tower wall and internals). The primary concern during air-freeing is to make sure that the tower is not isolated with steam inside because of the potential for the steam to condense and create a vacuum in the tower, which it may not be designed to handle.
On a start-up project observed by one of the authors, steam was venting from the tower top for a few hours. It was then observed during a walkthrough that the steam venting had stopped. The initial reaction was “great, we’re making progress. Let’s just make sure that gas is flowing into the tower by confirming there is no vacuum in the column”. One of the team opened a drain valve, put their hand by it, and almost had their hand sucked into the tower. At this point we were on our own in the middle of a large unit owned by a client company (we were licensor of the process technology, not the operating company), and were the nearest responders to a situation in which the tower could imminently collapse, resulting in millions of dollars damage and several months of delayed operation. The two options that we considered were: (a) run to the control room, and maybe five to ten minutes later get an operator to come out and fix things, during which time the tower might collapse, or (b) leave the drain valve open, introducing air into a system that might also contain fuel gas, potentially resulting in a fire or explosion. We opted for (b) followed by (a), and things worked out all right.
Safety issues identified
- Air-freeing might take longer with a divided wall if there is reason for flows of the purge steam to preferentially move up one side of the wall versus the other (or conversely, it may be possible to think that purging is complete by viewing steam at the tower vent, when in fact there is still some air on the side of the divided wall where the steam flow is restricted)
- A conventional steam-out procedure should consider the location(s) of the purge steam feed to the tower and the tower layout, to be confident that the purge is reaching both sides of a divided wall
Inadequate cleaning of debris from the tower
Incidents have occurred as a result of heavy scale or loose bolts, gloves, weld slag etc from initial installation activities that are inadvertently left behind when the tower is closed.
In the same distillation column mentioned in the previous story (which was used to separate a range of hydrocarbons from liquefied petroleum gas (LPG) to heavy gas oil), the tower was eventually air-freed, the unit was started up, and the feed mixture sent to the tower for separation. The tower and trays were constructed of carbon steel and there was some scale present in the system.
Once bulk hot liquid started flowing through the tower, all the debris, (including scale and “leftover components” such as nuts, bolts etc) was washed to the bottom of the tower and the bottoms pumps. The pumps were protected with suction strainers, but because of the huge amount of debris, the strainers then became plugged only seconds into operation. In order to clean the strainers, the pumps had to be shut down, the strainer isolated, the line opened, the strainer removed and cleaned with a steam hose, then reinstalled, line reopened, and pump restarted. This process was difficult enough for the operators to keep up with given the rapidity of strainer pluggage. But in addition, the process fluid at the bottom of the tower was above its autoignition temperature, and there was not enough interval between the strainers plugging on adjacent pumps to allow the system to cool down before removing the strainer for cleaning. Therefore, the operators were forced to open up the strainer pipe segment and deal with a fire that started when the strainer contents contacted the air. They managed this perilous activity by having people present with a steam lance to snuff the flames and cool the equipment, while someone else cleared the debris from the strainer. This activity took hours, but was completed without anyone being hurt. It would have been preferable to avoid the problem in the first place by having someone simply hose down the debris from the tower before the tower was closed.
Safety issues identified
- With dividing wall towers, there may be more opportunities for loose debris to accumulate in undetected locations
- Special packing shapes or high-efficiency valve trays may be more sensitive to residual debris accumulation from fabrication, although those applications are also presumed to be more likely to use stainless steel or other materials of construction that would reduce scale formation
Materials of construction
New equipment is typically designed to withstand the combination of thermal and chemical pressure, and other stresses that are anticipated for its operation. It is not uncommon for materials to be provided that do not meet the specification. There are simple field tests that can validate the materials of construction including portable X-ray diffraction devices for positive material identification.
Generally, incorrect materials that sneak through any screening/testing manifest themselves as problems during the normal operation. This may result in corrosion/disappearance of the component, and the ineffective separation that results. Usually there are no direct safety issues unless the outer wall of the tower, or connections to the tower, are made of incorrect materials.
We do know of a case where this issue arose because of the use of a dividing wall. The system was processing a mixed naphtha stream that contained residual fluorides from an upstream reaction. Unexpectedly, condensation occurred on a dividing wall. This does not normally occur in a traditional distillation where there is no wall since the tower shell is continually swept with liquid. The result was that the dividing wall corroded faster than the shell. The dividing wall was not the pressure boundary, so there was no safety implication from the corrosion itself. However, the design team should consider the potential consequences of such a breakdown of a dividing wall.
Safety issues identified
- More complex systems have a greater opportunity for incorrect materials to be used
- Unexpected material compatibility issues, such as the condensation concern described above
Liquid filling the tower
During a start-up operation in particular, it is possible to literally “flood” (liquid fill) a tower, as opposed to the more textbook version of flooding (having too much liquid/vapour traffic), which we discuss later. Perhaps the most well-known example of this occurred in 2005 at BP’s Texas City Refinery which resulted in 15 fatalities. During the start-up of the tower, an initial inventory of hydrocarbons was intentionally created in the tower bottom to allow for liquid/liquid traffic to be established when warming the tower later. However, the board operator failed to open the column bottoms line to tankage and was misguided by a level reading. The level instrument was a displacer-type device and was designed, in association with its transmitter, to measure the liquid level in a 1.5 m span such that 100% of its calibration corresponded to some 3.1 m in a tower that was 50 m tall. The apparent reduction in level was a result of the fluid at the base of the tower being at a higher temperature and therefore lower density within the displacer level device, which did not have temperature compensation. As the bottoms temperature in the column increased, the density fell, which was reflected by the apparent reduction in indicated level from 100% to 80%. The displacer level device no longer measured the level in the column but was responding instead to changes in the density of the fluid. Having a reading of less than 100% on the bottoms instrument suggested that more liquid could be added safely to the point where eventually the tower was completely liquid full. Once more heat was put into the tower, this liquid expanded and built pressure which was relieved through relief valves and sent to an atmospheric blowdown stack. There were problems with other, independent high-level alarms: one was not working and the other was ignored, as it was normal practice for this to be in alarm during start-up. The blowdown stack was designed to handle relief valve discharges of vapour. In this case, the bulk hot liquid discharge was vented from the top of the stack, fell to the ground, and found an ignition source.
In addition to the Texas City accident, several other incidents have been caused where level instruments confuse operators as they are responding to fluids with a density outside design parameters, either due to temperature or composition variations.
Safety issues identified
- More level gauges could become faulty or be misinterpreted – particularly with dividing wall columns
- More complex systems have greater potential for operators to misdiagnose what is going on in the column
- Operators may have less practice in bringing upset systems back to stability – particularly where they have a higher level of automation, such as with multivariable predictive control systems
Normal operation
Fouling
Distillation columns perform best when the feedstock is clean and free from particulates. However, this is not always the case and sometimes there is a scale buildup in the column. There have been cases where limited scale buildup has not been a problem with conventional tower internals (eg standard saddle packing). However, when this has been replaced with a higher efficiency packing, the scale buildup over time does become an issue and can affect distillation performance.
Safety issues identified
- While reduced efficiency in isolation would not be an issue, a continued buildup could lead to higher pressure drop and potentially unstable column operation. Column pressure relief systems are typically located at the top, so increased bottoms pressure may bring conditions closer to design limits than for conventional packing. Good operating practices should prevent issues with overpressurisation
- There are increased risks associated with isolating, decontamination, and opening-up process equipment for maintenance, so if the column requires more regular shutdown and decontamination for cleaning of packing, this may increase the overall operational risk
Flooding
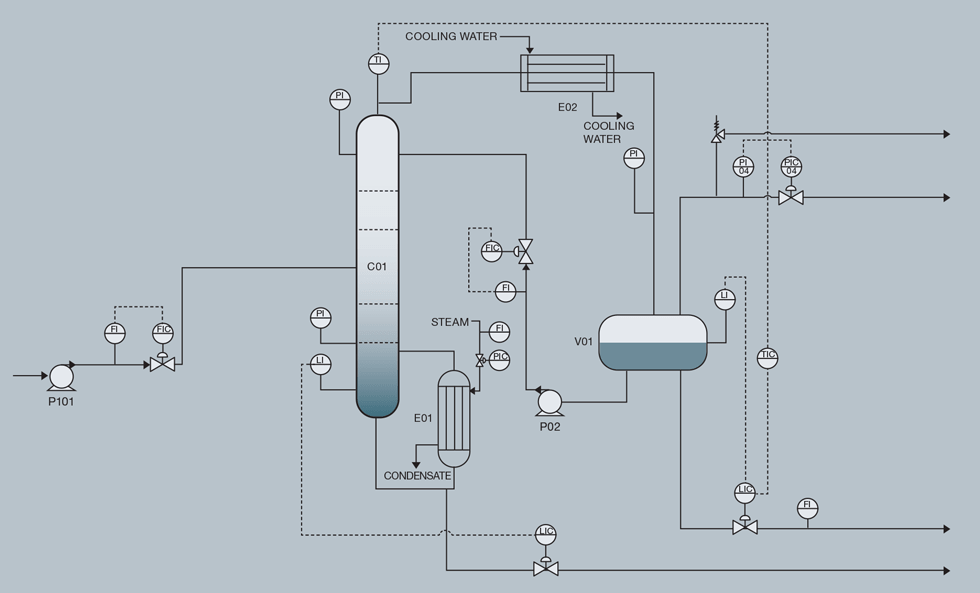
Flooding occurs when the upward flow of vapours and/or downward flow of liquid increases to a point at which liquid can no longer flow down the column. Such an event occurred at a plant not long after initial commissioning. In any start-up on new plants, it takes time for the board operators to get a “feel” for how equipment operates and responds to changes in setpoints. This can be more prevalent at facilities with less experienced operators.
Even so, experienced operators can create a flooded tower. A distillation arrangement including overhead condenser, receiver, reflux, and bottoms reboiler loop is shown in the figure opposite.
The typical sequence of events that leads to flooding of a distillation column is as follows:
1. Overhead and/or bottoms product compositions are off-spec for whatever reason.
2. Initial operator instinct is “Hmm; it looks like we may need more reflux to generate more liquid traffic”. So, the operator adds more reflux.
3. This quenches the tower, and doesn’t help the bottoms product quality, so the next instinct is to add more heat to the reboiler.
4. This doesn’t help either, so Steps 2 and 3 are repeated until the tower confronts other issues such as high pressure and unstable operation.
In one particular commissioning case, no progress was being made to resolve issues, so it was decided to give up trying to fix the situation and instead opt to just crash the column (stop heat) and rebuild from scratch. Fortunately, that was a workable option without causing operational issues because commissioning was underway and the tower was not yet fully integrated with the rest of the plant.
A flooding situation generally is not a safety concern, except if it progresses to the point of overpressurising the system, at which point the tower should be protected by pressure relief valves.
Safety issues identified
Divided wall column:
- There may be more of a tendency to inadvertently create a flooded condition on one side of the wall in order to optimise performance on the other side of the wall
- The added complexity may require a greater degree of knowledge and understanding by the operators on how to bring the column(s) back to more stable operation
Concentration buildup
Some distillation operations, particularly in which non-ideal solutions are involved that lead to low or high-boiling azeotropic mixtures, can result in concentration of a particular component on a certain tray or level within the column. This could lead to problems with materials of construction or, in the case of certain compounds, chemical stability issues.
Safety issues identified
- Hybrid distillation schemes, for example extractive distillation, may lead to a buildup of concentration of certain components in a particular area
- Dividing wall columns may have similar issues with unexpected buildup in certain locations
Poor/erroneous controls
Controls for distillation systems can be relatively simple, but despite that there have been several incidents associated with overfilling columns due to faulty controls and/or maloperation (eg BP Texas City, 2005, and Milford Haven Refinery, 19941). Faults with level instruments are sometimes difficult to diagnose and increasingly complex systems can make it even harder.
Safety issues identified
- Hybrid and dividing wall columns add to the complexity and may make it more difficult for operators to understand what is going on inside the column(s)
- The more advanced control systems are intended to aid the operator in areas such as safety, efficiency, and control of upset conditions. However, when experiencing abnormal operations, there are potential issues that the control system has not been designed to manage. In addition, operators may lose some of their troubleshooting skills as these are not practiced as often due to the control system doing such a great job most of the time
Feedstock change/contamination
There are cases where a chemical that is completely foreign to the main process flow enters the tower. One potential scenario is a leak or failure of a tube in a shell-and-tube heat exchanger in the feed preheating or tower reboiling system, leading to a heat medium such as steam entering the system. This is commonly disruptive to the normal operation of the tower; in some cases, the contaminant flashes upon entering the tower, resulting in trays or other internals being displaced and causing an extended shutdown.
Safety issues identified
- Where the technology involves a higher pressure drop, or perhaps even with a similar pressure drop, the consequences could be more severe than in a standard sieve-tray or packing-type tower. The process hazards review should recognise the potential for obvious contamination events and ensure that the equipment can sustain such events without catastrophic failure
Breaking of sight glass/other loss of containment
Loss of containment is a hazard present on all processes. In distillation columns, this might involve breakage of a sight glass (eg being accidentally hit with a wrench) or other equipment located near the bottom of the tower. For example, a field operator could inadvertently cause damage by using the equipment as a convenient platform for climbing to reach an instrument, valve, or other poorly placed device.
Many instruments will be connected by small-bore piping (although these should be avoided), potential weak points susceptible to damage by abuse or other failure modes. There are many other possible loss-of-containment scenarios, including improper closure/gasketing of flanges, long-term corrosion etc.
Safety issues identified
- A greater number of measurement devices connected to the column(s) provides an increased potential for failure
Failure of column intervals
Several industry accidents have occurred in which packing support grids have failed or trays have become detached from their supports. One situation occurred where a packed column was not operating efficiently and various investigations were undertaken. The column was then opened up for inspection during a turnaround. Once the column was completely empty, the cause was obvious: all of the packing was sitting in the sump following a failure of the support grid.
In some cases, failure can also be caused by one or multiple severe flooding incidents, where the packing (and support grid) has been lifted. Other failure cases have occurred due to corrosion of the support grids or associated attachments.
Safety issues identified
- For dividing wall columns, it may be more difficult to control temperature/pressure surges between column sections
- Advanced internals may be susceptible to unexpected corrosion phenomenon or more susceptible to flooding (being bounced)
- Pressure relief systems are typically located on top of the column, or the reflux drum. If the column internals fail and block the column, this may compromise the relief capacity, leading to overpressure and potential rupture of the column. Note: hazard studies at the design stage should ensure the relief system is always available and cannot be isolated, especially with the more complex distillation designs
An additional risk with distillation columns is where they are susceptible to the formation of deposits of pyrophoric compounds such as iron sulfide during operation. There have been multiple instances of internal fires caused when such compounds were present

Shutdown
Emptying and decontamination for maintenance
Safety issues when emptying and decontaminating distillation columns for maintenance are not significantly different to those associated with other process vessels. Equipment has to be properly isolated (disconnected prior to vessel entry), purged with inert gas, and possibly steamed out to remove residual flammable materials before air is added. However, an additional risk with distillation columns is where they are susceptible to the formation of deposits of pyrophoric compounds such as iron sulfide during operation. There have been multiple instances of internal fires caused when such compounds were present after air was introduced to the column, despite having been steamed out and/or inerted. Typical preventive measures include a thorough washing operation and sometimes a chemical decontamination process. It is nonetheless prudent to monitor the system for combustion when air is introduced, and be prepared for effective response should there be residual pyrophorics that the decontamination process did not remove.
Prior to entry of personnel, the column has to be adequately isolated, ventilated with air, and tested at multiple locations to ensure it is safe to enter. If entry involves disturbance of deposits, repeat gas testing is required at suitable intervals. Where it is not possible to guarantee the air purity, use of breathing apparatus such as an air-fed mask should only be used in exceptional cases and under carefully controlled conditions.
Safety issues identified
- For advanced packing, especially structured packing, there may be a greater tendency to form pyrophoric compounds. Chemical decontamination should be considered
- For dividing wall columns, there may be more places for pyrophoric compounds to become lodged
- For vessel entry involving dividing wall columns, it may be more difficult to test the atmosphere in all locations. Furthermore, there may be greater challenges for safe access and for emergency escape from a dividing wall column, and procedures should be rigorously tested to ensure they are practical and safe
Conclusions
This article provides examples of potential safety issues with distillation systems and has identified where special consideration may be required for some of the novel designs described in previous editions of TCE. Although no completely new safety phenomena related to the advanced distillation methods have been identified, the added complexity may aggravate known issues. There will be other matters that may require further analysis, and these should be identified using existing process safety techniques such as process hazard analysis, change management etc.
References
1. https://www.hse.gov.uk/comah/sragtech/casetexaco94.htm
Disclaimer: This article is provided for guidance alone. Expert engineering advice should be sought before application.
This is the sixth and final article in a series helping chemical engineers to take advantage of opportunities to improve the efficiency of distillation operations. To read more, visit the series hub at: https://www.thechemicalengineer.com/tags/distillation-improvement-opportunities
Roger Stokes and Michael Moosemiller welcome feedback from sites where problems have arisen.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.






