Dermot Manning and colleagues at ICI – Plastic Fantastic
The commercial success of PE starts with Reginald Gibson, Eric Fawcett, Michael Perrin and Dermot Manning at ICI. Claudia Flavell-While tells their story
POLYETHYLENE (PE) – or polythene as it’s still known to many – is, quite literally, everywhere. Low-density polyethylene (LDPE) shopping bags are used to carry home clingfilm (also LDPE), freezer bags (very low-density polyethylene, or VLDPE), bubble wrap and Tupperware food containers (linear low density polyethylene, or LLDPE), milk bottles, detergent bottles, margarine tubs and plastic toys (all made from high density polyethylene, or HDPE).
Meanwhile your water and gas may well reach you via a pipe made of medium density polyethylene (MDPE); the water pipes inside your home, if they aren’t copper, are almost certainly cross-linked polyethylene (PEX); and if you’re unlucky enough to have needed a hip or knee replacement operation, the articulated section of the joint is probably made from ultra-high molecular weight polyethylene (UHMWPE).
In short, PE is an incredibly versatile material, which has made it the world’s most important and most produced plastic, with production running to around 80m t/y.
As one might expect, chemical engineers had a crucial hand in its discovery and commercialisation.
PE was discovered twice, each time by accident. The German chemist Hans von Pechmann was the first to synthesise it, in 1898, as an unexpected result of heating diazomethane. In 1933 it was discovered again by two research chemists, Reginald Gibson and Eric Fawcett, working at ICI Alkali’s research laboratories in Winnington, UK. A third chemist, Michael Perrin, made the crucial breakthrough in understanding PE and its production that turned a fluke discovery into a reproducible reaction. But it was a chemical engineer, Dermot Manning, who built the high-pressure research reactor that made the experiment possible, as well as the larger reactors subsequently needed to move to pilot, demonstration and full-scale production.
A mystery solid
The discovery of PE was the result of a forward-looking research programme coupled with, as is so often the case, a dose of sheer luck. In the early 1930s, with the world in the grip of the Great Depression, ICI’s joint research manager Frank Freeth directed the company to work on novel reactions, without any link to existing products and reactions. The Alkali Division responded with a high-pressure research programme, investigating reactions at 1,000–20,000 atm.
Fawcett and Gibson got to work, initially investigating high-pressure reactions of liquids but quickly moving on to gases. They initially used a 50 ml high pressure reaction vessel supplied by A Michels at Amsterdam University, but soon replaced it with an improved version built by the high pressure team’s resident engineer, Manning.
In March 1933, the research had moved on to a reaction of ethylene and benzaldehyde. On 27 March, following an experiment that had subjected the mixture to a pressure of 1,900 atm at 170ºC, Gibson and Fawcett noted an unexpected pressure drop and a mystery “waxy solid” in the reaction vessel – polymerised ethylene.
The strange new substance, however, proved to be difficult to handle: most experiments produced not a waxy solid but a loud bang, hydrogen and carbon. Despite continued efforts, attempts to replicate the experiment were largely unsuccessful, though it remained a mystery why the reaction was so unpredictable. Concerned that the frequent violent decompositions would one day result in something more serious than a loud bang and a cloud of soot, ICI eventually shelved the project, and Gibson and Fawcett moved on.
The missing ingredient
In late 1935 and following a number of improvements to the high pressure research equipment, ICI’s research director Michael Perrin decided to review Fawcett and Gibson’s work. Fortune was kind: the first recorded experiment, on 20 December 1935, produced not an explosion but a white solid: polyethylene.
However the tendency for ethylene to rapidly and violently decompose remained. It wasn't until an Oxford consultant, Paul Hinshelwood, suggested that the researchers look at potential interactions with oxygen, that the mystery of why the reaction was so temperamental was finally solved. As the research team discovered, the polymerisation reaction requires a small amount of oxygen to supply the radicals needed to initiate the process.
Furthermore they found that at the time, it was common practice to return gas cylinders for refilling with their valves open. This meant that the cylinders could contain anywhere up to 1 atm of air – indeed the supplier, BOC, only guaranteed the gas to be 95% pure. The remaining 5% often included a quantity of oxygen, though exactly how much oxygen was present varied considerably. The quantity of oxygen mixed with the ethylene has a huge impact on the reaction: if it’s completely absent, there is no reaction; at 0.002% the reaction is stable, but if the concentration reaches 2% or more, the mixture explodes.
By pure chance, Gibson and Fawcett and later Perrin had for their early experiments picked ethylene canisters containing just the right level of residual oxygen.
Understanding the role played by oxygen provided the missing link to the research, which started to yield much more predictable and interesting results and ICI soon realised that it had stumbled upon a material with considerable potential.
Designed for pressure
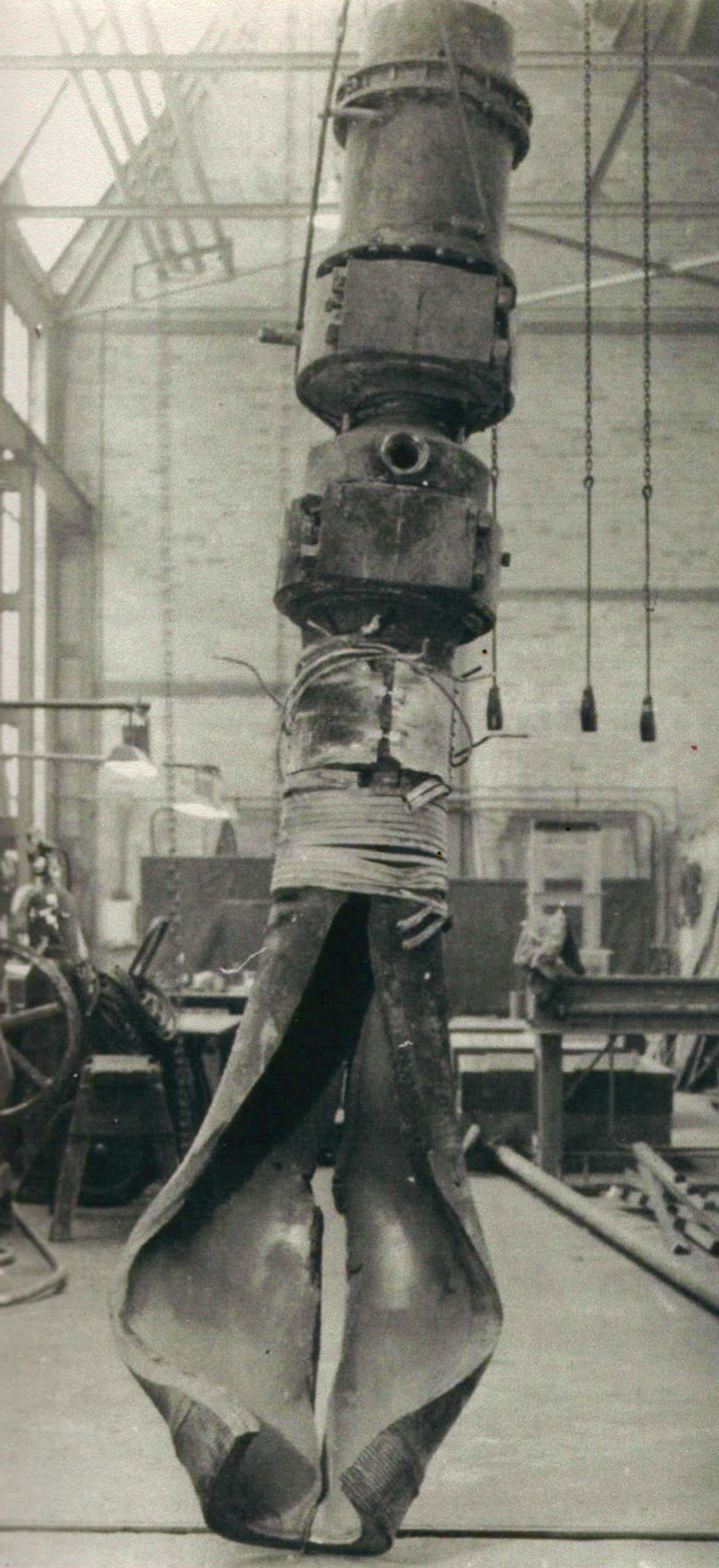
High-pressure chemistry was still a fairly new area of research during the 1930s and designing and building the reactors was not straightforward. “The most important problem was how to make vessels and other equipment strong enough to contain a fluid with a reasonable margin of safety between the required maximum operating pressure and the bursting pressure,” writes ER Ellis in his book, Polythene came from Cheshire. Manning noted that while a brittle steel would fail as soon as the internal pressure reached bursting point, a ductile steel would dilate first, which would give the reactor a reserve against accidental overpressure.
Sealing a gas in at pressures of 1,000 atm and more is equally difficult and the lens ring commonly used becomes unsuitable at 300 atm and more. Manning overcame the problem with a self-sealing wave ring seal which used the internal pressure of the reaction vessel to seal the wavy circumference into their seats.
With the renewed interest in PE in 1936 came the demand for larger reaction vessels and by the end of the year, Manning designed and built a 750 ml vessel featuring a blow-down valve through which PE could be ejected from the reaction vessel while a feed of ethylene gas maintained the reaction, proving that the reaction could take place in a continuous process.
Pilot trials involving a 9l continuous reactor at Wallerscote followed. Unintended decompositions of ethylene continued to be a hazard and the reactor’s safety valve remained in regular use, but other aspects of the process were much improved. For example, the addition of a heated hopper and separation system allowed operators to capture and recycle the unconverted ethylene that until then was simply vented to atmosphere.
In July 1939, the pilot plant was upgraded with the first of two 50 l reaction vessels, also built by Manning. The 100 t/y plant was formally moved into full-scale production on 1 September 1939 – the same day that Germany invaded Poland.
Change in application
Early applications for PE made use of its high dielectric strength, low loss factor and moisture resistance and early interest came from telecommunications companies, who wanted to use it to insulate transatlantic cables.
But the outbreak of World War II meant that another use took precedence: insulating radar cables. Almost all the 4000 t of PE produced between 1939 and 1945 was used to insulate radar cables, and the use of airborne radar proved to be a critical advantage in the Battle of the Atlantic, helping British supply ships avoid German submarines. PE was much lighter than other insulating materials, which in turn enabled the British forces to create radar systems light enough to place on fighter planes.
Indeed the discoverer of radar, Sir Robert Watson-Watt, said: “The availability of polythene transformed the design, erection, installation and maintenance of airborne radar from the almost insoluble to the comfortably manageable.”
Following the end of the war in 1945, there was a brief lull in demand for polyethylene. Its use in radar meant that ICI had had to keep its existence secret, so at the end of the war it took some time to establish peaceful applications.
Saved by Hula-Hoop
Early PE was notoriously soft and had a low melting point, making it unsuitable for any applications involving heat. This problem was only overcome when German chemist Karl Ziegler developed catalysts that resulted in low-branching straight-chained PE which was much more rigid and had enough temperature resistance to handle boiling water – HDPE. HDPE was an instant success and companies rushed to use and produce it.
But disaster struck when it was discovered that the early HDPE was brittle and prone to cracking after a few months. The solution was to allow the formation of a small number of side chains. But what were PE producers to do with the huge quantities of brittle HDPE they’d already produced and stockpiled?
The answer came from an unexpected quarter: toy manufacturer Wham-O and the late 1950s Hula-Hoop craze. Consisting of simple tubes of extruded PE, the brittleness and cracking was much less of a problem here. Soon the stockpiles were consumed, the process adjusted, and PE was finally and irrevocably on the path to becoming the world’s most important polymer.
Originally published in November 2011
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




