Water purification using sunlight and hydrogel
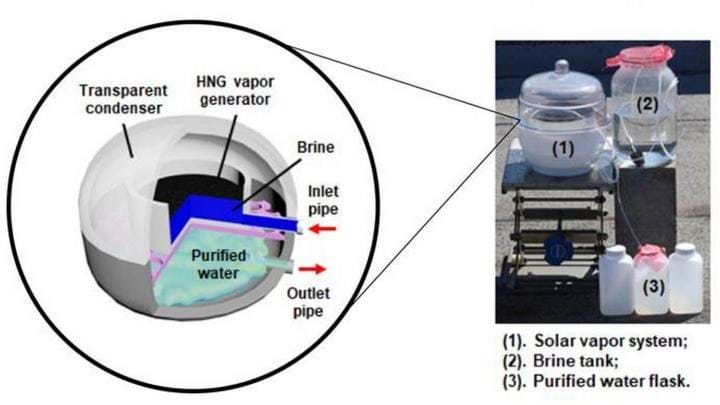
A WATER purification method has been developed that uses sunlight along with an inexpensive gel, rendering even high-salinity water safe to drink.
The availability of clean drinking water is a major problem in some developing nations and can also be an issue after bad storms or natural disasters that disrupt the drinking water supply. A team of researchers, led by Guihua Yu at The University of Texas at Austin, have now developed a method for water desalination that only needs natural levels of sunlight and an inexpensive hydrogel.
The hydrogel-based solar vapour generator uses sunlight to evaporate water and the water vapour is then pumped to a condenser to make fresh water. The hydrogel significantly increases the volume of water evaporated compared to conventional methods, and it only needs naturally-occurring levels of sunlight, unlike others which need to focus the light using costly optical instruments.
“Water desalination through distillation is a common method for mass production of freshwater. However, current distillation technologies, such as multi-stage flash and multi-effect distillation, require significant infrastructures and are quite energy-intensive,” said postdoctoral researcher Fei Zhao. “Solar energy, as the most sustainable heat source to potentially power distillation, is widely considered to be a great alternative for water desalination.”
The hydrogel is based on polyvinyl alcohol (PVA) and polypyrrole (Ppy). The PVA chains in the gel stop the heat loss from the water via convection, which is a problem in other designs. In addition, the gel is composed of water pathways consisting of gaps, micron channels, and molecular meshes, which enable the rapid replenishment of water in the gel. The Ppy solar absorbers in the PVA allow for the solar energy to be directly delivered to the water in the molecular meshes.
The energy efficiency of the gel was 94% under the equivalent of radiation from “1 Sun”, or 1 kW/m2. In other systems, an efficiency greater than 90% can only be achieved with highly concentrated solar radiation above 4 kW/m2. The temperature of the gel increased from 21.3 to 37.6oC within 500 s and then increased further to 41.3 oC within 1 h. The bulk of the water remained at 21.3 oC after 1 h.
The team tested their method on three simulated brine samples by dissolving salt in the water to represent the Baltic Sea (0.8%), World sea (3.5%) and Dead Sea (10%). The salination decreased by around four orders of magnitude, bringing the levels below those approved by the World Health Organization (1‰). They also used a real sample from the Gulf of Mexico and found that the concentrations of all four primary ions (Na+, Mg2+, K+, and Ca2+) were reduced below the level usually obtained with membrane-based techniques.
“Our outdoor tests showed daily distilled water production up to 25 L/m2, enough for household needs and even disaster areas,” said Guihua Yu, who led the research. “Better still, the hydrogels can easily be retrofitted to replace the core components in most existing solar desalination systems, thereby eliminating the need for a complete overhaul of desalinations systems already in use.”
Nature Nanotechnology http://doi.org/gc95sn
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




