Understanding why fracking wastewater contains radioactive waste
RESEARCHERS at Dartmouth College, US, have released a study explaining the transfer of radium to wastewater during hydraulic fracturing for oil and gas extraction. An understanding of the mechanisms involved could lead to the development of strategies to mitigate wastewater production.
During hydraulic fracturing, or fracking, fluid is pumped underground at high pressure to break apart rock and create fractures which oil and natural gas can flow through. A common practice is to use “slick water”, which is a combination of water, a proppant – typically sand – and a mixture of chemicals. After the hydraulic pressure has been dropped the proppant holds the fractures open. Friction reducers, usually a polyacrylamide, are a critical component added to increase fluid flow. Other chemicals, such as biocides, surfactants, and scale inhibitors can also be added.
Once the pressure has been dropped slick-water returns to the surface as wastewater which is salty and highly toxic. It contains toxins such as barium (Ba) and radioactive radium (Ra). As Ra decays it releases a cascade of other elements, such as radon, that collectively generate high radioactivity.
Fracking is a widely-used method for oil and gas extraction. Due to fracking, US production of oil and natural gas has increased dramatically over the past decade. An understanding of the mechanics of Ra transfer during fracking could help researchers to overcome the associated challenges.
Taken together, the twin studies published in Chemical Geology are the first to help to explain the mechanics that result in the high salinity and Ra content of fracking wastewater. Before the release of these studies researchers were uncertain if radioactive Ra came directly from the shale, or if it came from naturally occurring brines present deep in Pennsylvania Marcellus Shale.
An earlier study carried out at Dartmouth found that barium, which is in the same group of alkaline earth metals as Ra, reacts to fracking in similar ways.
Mukul Sharma, head of the research project and professor of earth sciences at Dartmouth, said: “I think that water-rock reactions are key to generating the peculiar chemistry of the brines recovered after fracking and during the production phase (wastewater). We have shown this for Ba and Ra that are two geochemically related elements but whose sources are quite different in the black shale.”
The first paper
The researchers focused on rocks from the Marcellus Shale, a geological feature in Pennsylvania and New York where fracking is being carried out.
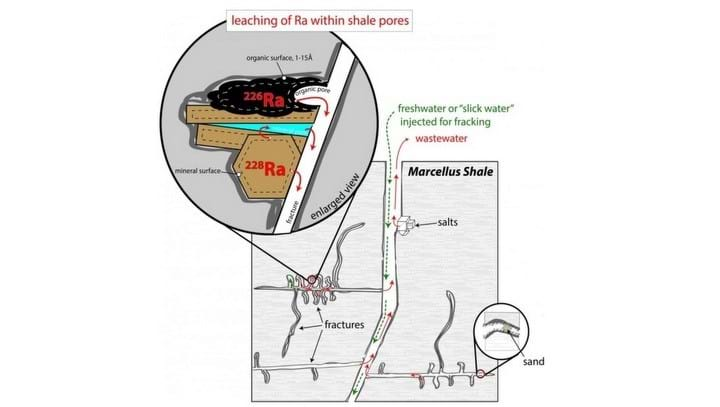
The researchers focused on rocks from the Marcellus Shale, a geological feature in Pennsylvania and New York where fracking is being carried out.
The leachable Ra within Marcellus Shale was found to originate from two distinct sources. Thorium-rich clay mineral was found to be the source of 228Ra, which accounted for 4% of the Ra in shale. The Ra from this source was found to be labile and water accessible. The other source of Ra was a uranium-rich organic phase. The organic phase was the source of exchangeable 226Ra, which accounted for 14% of the Ra in shale.
Leaching experiments showed that Ra extraction from these phases is rapid. The researchers also discovered that extraction increased with salinity. Pure water was unable to produce wastewaters with similar radioactivity to Marcellus wastewaters, but those with increasing concentrations of ionised calcium (Ca2+) approaching 1 mol produced comparable radioactivity.
The second paper
The second research paper described the Ra transfer mechanics. The model attributed the extreme salinity and Ra content of Marcellus wastewaters to the progressive, hydrologic enrichment of injected fluids during hydraulic fracturing. Interaction of injected fluid with rock results in a gradual increasing of the Ca2+ concentration and the increasing Ca2+ concentration favours the desorption of 226Ra from organics.
Joshua Landis, a senior research scientist at Dartmouth and lead author of the papers, said: “There are two distinct phases in the Marcellus Shale that control Ra mobility; the phase that behaves similarly to what has elsewhere been described as ‘brine’ is inconsistent with the Ra isotopic composition of fracking wastewaters.
“To reproduce the Ra isotopic composition of wastewaters we require release of Ra from a second phase, an exchangeable phase of the rock. This occurs by sorption-desorption reactions between the increasingly saline fracking fluids and the rock within the shale pore network.”
Landis added that the physical process involved “allows extraordinarily low water-to-rock ratio during fracturing, where one litre of injected fluids appear to remove Ra from as much as 25 kilograms of rock before returning to the wellhead. The physical constraints on this interaction are not well understood.”
Mukul Sharma said: “To release Ba and Ra requires interaction of the rock with high salinity solutions. The detailed mechanism that leads to creation of these high salinity solutions in the first place needs to be elucidated. This is what we are working on now. Since water-rock interaction is a universal process the mechanism(s) leading to production of brines could potentially be applied to other situations such as oil-field brines.”
Chemical Geology: http://doi.org/gfbwbn, http://doi.org/cwwg
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




