Novel epoxide synthesis
RESEARCHERS at MIT have developed a novel method for epoxide synthesis which could offer a safer and more sustainable alternative.
Epoxides are ubiquitous, according to Karthish Manthiram, Assistant Professor of Chemical Engineering at MIT. They are used in the manufacture of a wide range of products, such as plastics, textiles, and pharmaceuticals, and they are intermediates in various other chemical products.
Current epoxide synthesis methods can involve the production of large amounts of carbon dioxide (CO2). Several epoxides are among the chemicals with top carbon footprints. For example, production of ethylene oxide, a common epoxide, generates the fifth-largest CO2 emissions of any chemical. Epoxide synthesis can also involve hazardous reagents, and environmentally undesirable waste products.
Taking inspiration from water oxidation, the team at MIT developed a more sustainable synthesis method. In water oxidation, water is split into oxygen, protons (H+), and electrons. The team at MIT decided to try and perform water oxidation, and subsequently attach the oxygen atom to an olefin, a precursor to epoxides. According to Manthiram, this was counterintuitive because olefins do not typically react with water. However, the reaction is possible when an electric voltage is applied.
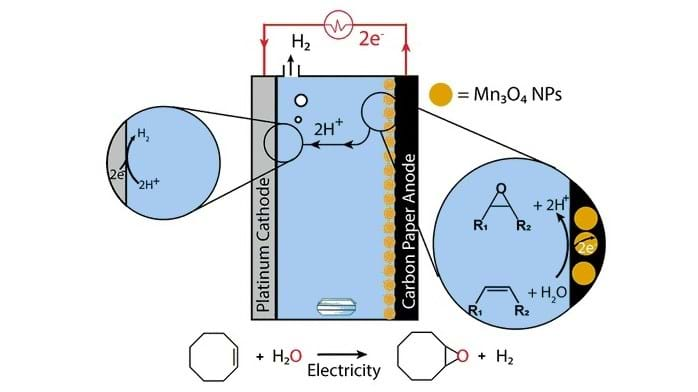
The researchers designed a reactor for the process. At the reactor’s anode, water is split, and then the protons and electrons generated flow to the cathode, where they are converted to hydrogen gas. Manganese oxide nanoparticles act a catalyst and enable incorporation of oxygen into an olefin, creating an epoxide.
The process can be employed at room temperature and pressure and eliminates production of CO2. In addition, epoxide synthesis is typically energy-intensive and most of the energy used is produced using fossil fuels. The team believe they could reduce the carbon footprint of the process further by using electricity from renewable sources, such as solar and wind, for epoxide conversion.
So far, the researchers have successfully produced the epoxide cyclooctene oxide using this method, achieving about 50% conversion over 4 hours. They are currently investigating how to adapt the method to generate other epoxides.
The researchers also seek to make the process more efficient, to enable it to be adapted for large-scale, industrial use. In the current study, about 30% of the electrical current applied was used for conversion. Researchers aim to double this.
If the developed process is scaled up, researchers estimate that ethylene oxide could be produced for US$900/t. Production using current methods costs US$1,500/t. Researchers believe that the cost of the novel method could be further reduced by improved efficiency. Generation of hydrogen could also contribute to economic viability.
“We are continuing to uncover new catalyst compositions which are even more effective for epoxidation. The development of efficient catalysts is essential for reducing the overpotential of the epoxidation reaction. We are optimising process conditions to improve the Faradaic efficiency and selectivity toward the epoxide. In addition, we are also designing reactors which will allow for a high flux of olefin epoxidation.”
The researchers are also working on using electricity to synthesise other chemicals.
“There are many processes that have enormous carbon dioxide footprints, and decarbonisation can be driven by electrification,” said Manthiram. “One can eliminate temperature, eliminate pressure, and use voltage instead.”
“Our proposed method is part of a broader toolkit for electrically-driven chemical synthesis, which can be more modular due to relaxed temperature and pressure requirements, enabling distributed chemical manufacturing. We envision a future in which small-scale chemical reactors which are close to the point-of-need will facilitate chemical reactions involving locally-available resources, including water and sunlight.”
The researchers have filed an application for a patent for their process.
Journal of the American Chemical Society: http://doi.org/c5vz
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




