Cyborg bacteria make chemicals from sunlight
“CYBORG” bacteria covered in tiny solar panels have been demonstrated to produce acetic acid from sunlight, water and air – providing a potential feedstock for biosynthesis of fuels and plastics.
The research, presented today at a meeting of the American Chemical Society in Washington, DC, could offer a method of harvesting sunlight for chemical bioproduction that is more efficient than plant-based photosynthesis.
Kelsey Sakimoto, who undertook the research at the US' University of California, Berkley, said: “Rather than rely on inefficient chlorophyll to harvest sunlight, I’ve taught bacteria how to grow and cover their bodies with tiny semiconductor nanocrystals.
“These nanocrystals are much more efficient than chlorophyll and can be grown at a fraction of the cost of manufactured solar panels.”
To do this, cultures of a nonphotosynthetic bacterium, Moorella thermoacetica, were fed cadmium and a sulfur-containing amino acid, cysteine. The bacteria then began to produce the inorganic semiconductor cadmium sulfide – a compound classically used in the early days of photovoltaic panels.
When exposed to light, these panels produce electrons that are utilised by M. thermoacetica, which produces acetic acid from carbon dioxide as part of its natural method of acquiring energy.
Benefits of this process include that modified bacteria operate at an efficiency of 80%, and that they are self-replicating and self-regenerating.
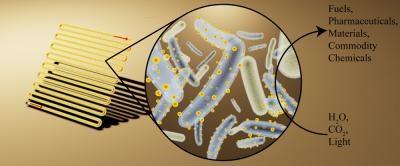
Sakimoto said in a press conference that the acetic acid was a good starting point for solar to chemical production, from which a range of engineered bacteria such as E. coli could continue its transformation into useful products.
He said that collaborators at the University of California, Berkley, had already used it as a feedstock without purification or further processing to produce butanol, the bioplastic polyhydroxybutyrate and a number of pharmaceutical compounds.
“Synthetic biology and the ability to expand the product scope of CO2 reduction will be crucial to poising this technology as a replacement, or one of many replacements, for the petrochemical industry,” Sakimoto said.
He added that superior alternatives to cadmium used in more modern photovoltaic systems, such as indium phosphide, are of interest for future research.
He suggested that these microbes capable of producing these compounds could potentially be found using bioprospecting techniques in environments such as clean rooms in microfabrication facilities.
PNAS: http://doi.org/f88d4x
Science: http://doi.org/f74rzf
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




