New process for creating biodegradable polyesters

RESEARCHERS have developed a new polymerisation process involving a light-activated catalyst that could be used to create biodegradable plastic.
Most plastics are made from a class of polymers known as polyolefins. These non-degradable polyolefins create a huge problem with plastic pollution, but biodegradable alternatives that match the properties of conventional plastics are challenging to develop due to the limited chemical structures of the alternatives.
Rong Tong, assistant professor in the department of chemical engineering at Virginia Tech, US, led a team of researchers in developing a new polymerisation process for polyesters which has the potential to replace polyolefins.
The arrangement of substituents along a polymer chain, known as tacticity, is key to controlling the thermal stability and crystallinity of the polymer. There are several different configurations possible; isotactic polymers have pendent groups that are all the same, syndiotactic polymers have alternating groups, and stereoblocks have blocks of alternating groups. Collectively, these are known as stereoregular polymers and they tend to have higher melting temperatures and improved mechanical properties over atactic polymers, where the units are arranged randomly.
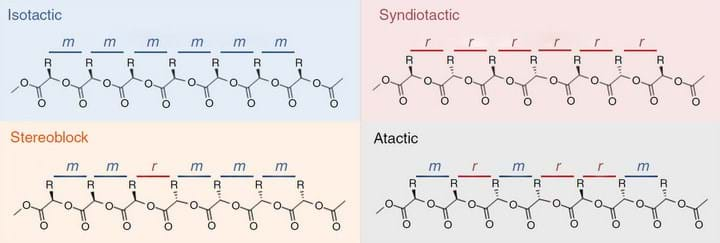
Tong and colleagues created stereoblock polymers using stereoselective ring-opening polymerisation (ROP). Stereoselective polymerisation occurs when one particular geometrical arrangement of atoms – known as a stereoisomer – is formed in greater amounts over another stereoisomer.
They used a versatile type of monomer called O-carboxyanhydride, which is derived from natural amino acids which make the monomer biodegradable. They combined a photoredox Ni/Ir catalyst – which uses a household bulb to start the reaction – with a stereoselective Zn catalyst to initiate the ring-opening polymerisation. The resulting stereoblock polymer has a high molecular weight, thermal stability and crystallinity, and can degrade in a basic water solution.
"If you use a regular catalyst, it doesn't have stereochemistry control, but we found that our catalyst can do that," said Tong.
Similar work had been performed previously with polylactide, but by controlling the stereochemistry, the researchers have been able to improve the physical and chemical properties of the resulting polyester.
"This polyester synthesis that controls the tacticity can provide a new library of polymer materials that we haven't had before," said Guoliang “Greg” Liu, an assistant professor in the department of chemistry.
The polymerisation process has currently only been demonstrated at lab scale, but the researchers hope that it can be used to create biodegradable plastics in the future.
"It would be our dream to see these degradable polyesters materialise in the marketplace, for both the plastic industry and biomedical application," said Tong.
Nature communications http://doi.org/gdcfqr
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




