First kerosene made from solar syngas
SYNGAS made from solar energy has been processed into kerosene for the first time, which researchers say could be used as jet fuel.

The syngas was produced by concentrating 3,000 ‘suns’, or flux radiation, of solar energy into a high-temperature thermal solar reactor at 1,500°C, causing thermochemical splitting of H2O and CO2 into H2 and CO.
The work was undertaken at ETH Zurich, Switzerland, as part of the five-partner SOLAR-JET project. In total, 700 L of syngas was obtained after 295 consecutive cycles of the 4 kW solar reactor. This was then refined into kerosene, using the Fischer-Tropsch method, by Shell Global Solutions in the Netherlands.
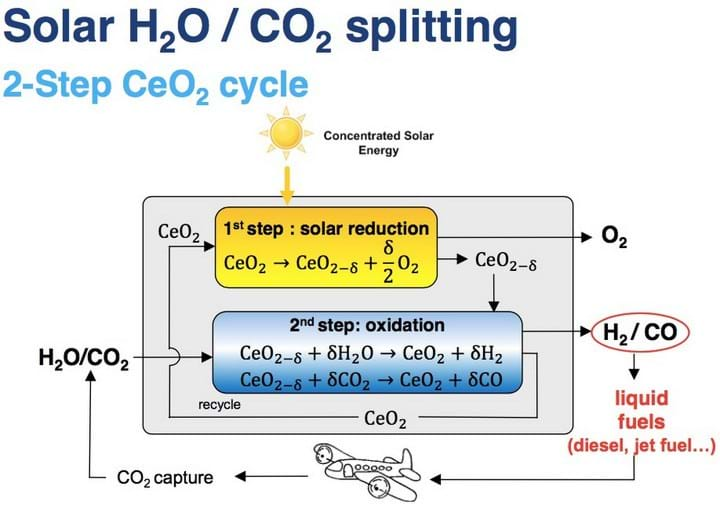
The ETH team say that the key component in the two-step H2O and CO2 splitting process is a reticulated porous ceramic (RPC) structure in the reactor. It is made of a material called ceria (CeO2) which facilitates molecule splitting.
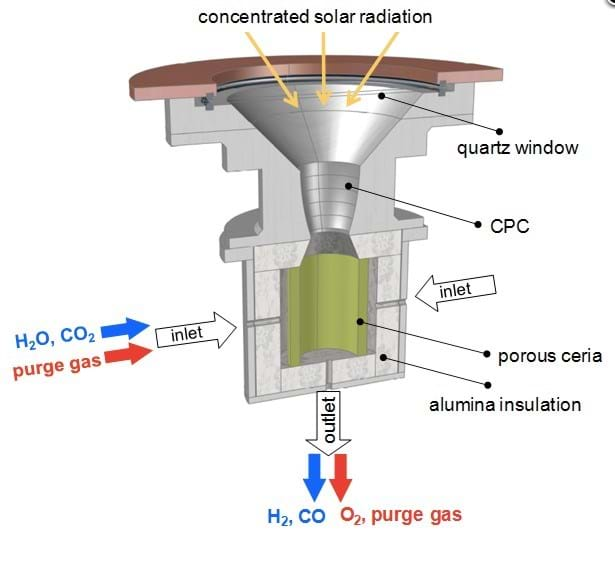
“Ceria is the state-of-the-art material,” said Philipp Furler, ETH researcher and leader of international solar chemistry research group SolarPACES Task II. “It has the ability to release a certain amount of its oxygen and then in the reduced state it has the capability of splitting water and CO2. In the first step, at 1,500°C, we release a fraction of the oxygen contained in the material, thereby a pure stream of oxygen leaves the reactor as a valuable by-product.”
The second step takes place at a temperature of around 1,000°C, when H2O and CO2 are introduced. In order to assume its initial state, the ceria must take up the oxygen it previously held. To do so, it splits the available H2O and CO2 into oxygen and H2/CO syngas, which can be extracted from the reactor.
This process was demonstrated, in an Energy & Environmental Science paper, to split CO2 into separate streams of CO and O2 with 100% selectivity, 83% molar conversion, and 5.25% solar-to-fuel energy efficiency.
The SOLAR-JET consortium hopes to use the solar-derived kerosene in jet fuel, as a fossil fuel replacement.
“I am very convinced that it will be the case that conventional fuels will be punished by CO2 taxes in the future,” said Furler. “This will be the main driver. And the big oil and gas companies are afraid of this situation. We believe that this is a future business.”
Aside from ETH and Shell, other project partners include combustion and high-temperature chemistry specialist DLR, mobility research association Bauhaus Luftfahrt (BHL), and management provider Arttic.
“Our long-term vision, and what we are following is that we will be extracting the CO2 from the atmosphere. This way, we are able to close the carbon cycle and to produce CO2-neutral fuels,” said Furler.
According to Andreas Sizmann, SOLAR-JET project coordinator at BHL, a solar reactor with a 1 km2 solar field could generate 20,000 L/d of kerosene, which could fly a large 300-body commercial airliner for about seven hours.
Furler has founded the ETH spin-off Sunredox to commercialise the technology. “We need the support of people with the know-how, and guys in the oil and gas industry have shown that they know how to massively scale up,” he said.
Energy & Environmental Science: http://doi.org/cgbj
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




