3D printing of living cells using in-air microfluidics

A TECHNIQUE has been developed that allows two fluids to be combined mid-air by using two jets of material. This can be used to encapsulate living cells for potential use in tissue engineering.
Lab-on-a-chip microfluidics uses tiny fluidic channels that limit the speed at which droplets can leave the chip. The typical speed is in the microlitre per minute range, so that filling a cubic centimetre would take around 17 hours. Researchers at the Netherlands’ University of Twente have developed a new method, called in-air microfluidics, that combines micrometre-sized streams of liquids mid-air to fill a cubic centimetre volume within a matter of minutes.
The jets can be made of different types of fluid that react chemically or physically, so that the collision between them can create new materials. The material can then solidify to build a 3D structure.
Two nozzles are used to create the flow of liquids. A droplet train can be ejected from the first nozzle and will combine with a continuous stream from the second nozzle, known as “drop-jet” mode. Alternatively, both nozzles can create jets, known as “jet-jet” mode, and this can be used to create fibres.
Droplets can be encapsulated by ensuring that the surface tension of the two liquids is different. The surface tension of the jet can be reduced by adding ethanol so that when the droplet and the jet collide, the low surface tension jet spreads around the high surface tension droplet. The encapsulation occurs in the air around 100 ms after the impact.
The sizes of the droplets can be varied by adjusting the diameter of the nozzle. The shape can be controlled by changing the velocity ratio between the jets. Elongated particles can be created by increasing the velocity of the jet while keeping the velocity of the droplets the same.
The shell around the droplet can solidify in the air so that the encapsulated droplets can then be stacked to create a 3D structure. The droplets will stick together without any air bubbles between them.
The researchers used the droplets with the solidified shells to create a hollow cylinder by depositing the droplets on to a rotating surface. The modular freeform can have different functions by changing the composition of the fluids, for example the structure could be a liquid-filled foam or a multimaterial solid.
The in-air technique can also be used to fill a mould if the droplets solidify mid-air, but the shells don’t solidify until the mould has been filled. The team used this method to fill a bone-shaped mould with human mesenchymal stem cells (MSCs) encapsulated with alginate.
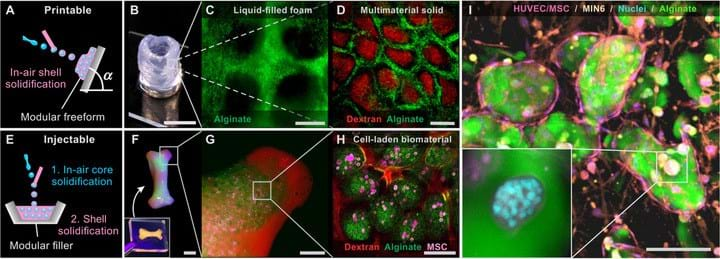
By creating multicellular and multimaterial 3D structures, this type of modular biomaterial has the potential to be used in tissue engineering. There is also a hand-held setup of the nozzles which could benefit tissue engineers and surgeons for fabricating biological tissue.
Science Advances http://doi.org/cj9g
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




