Understanding Flammable Gases: Composition and Safety

Hazardous environments consultancy Armadex highlights the importance of safety data sheets (SDSs) when it comes to providing information on the handling of flammable gases, their hazards, and emergency measures
FLAMMABLE gases occur in various forms, from naturally occurring methane to pure substances like hydrogen and evaporation products like acetone. Handling these gases requires a deep understanding of their properties and safety limits, making the study of safety data sheets (SDS) essential for anyone working with these substances.
Official definitions and categories
The Globally Harmonized System (GHS) defines flammable gases as those with a flammable range under standard conditions of temperature and pressure (STP). Gases remain gaseous under normal atmospheric conditions, while vapours are the gases emitted from volatile liquids.
Gases are broadly classified into three groups:
- Oxidisers: These are not flammable but support combustion (eg oxygen and bromine gas)
- Inert gases: These do not react with other substances and include noble gases like helium and argon, as well as carbon dioxide
- Flammable gases: These can ignite and lead to fires or explosions under the right conditions
Flammability and explosion limits
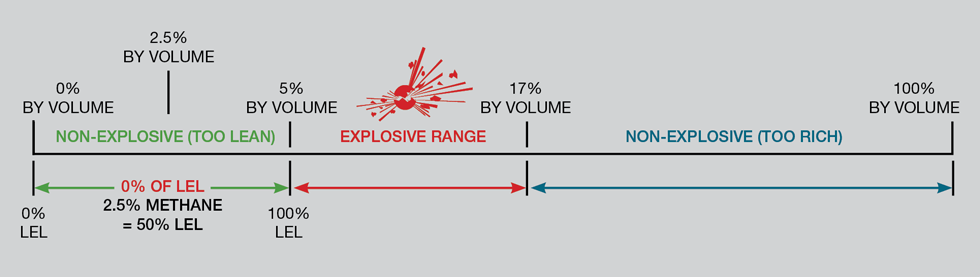
A critical aspect of working with flammable gases is understanding their lower explosion limit (LEL) and upper explosion limit (UEL). These limits define the concentration range within which a gas can form an explosive atmosphere when mixed with air and exposed to an ignition source. For instance, hydrogen gas becomes explosive at concentrations above 4% and below 76% in air.
Consider this real-life example of UEL and LEL. The survival of over 60% of passengers in the 1937 Hindenburg disaster, where a hydrogen-filled airship fell 61 metres, is attributed to the nature of the hydrogen gas leak. The gas, upon leaking, ignited and rose, taking most of the heat upwards, away from the passengers. Although the airship’s material burned, the hydrogen gas itself was too concentrated to cause an explosion. It only burned where it met the air. Had it exploded, there likely would have been no survivors.
Characteristics of notable flammable gases
Ammonia
Used in fertilisers and as a refrigerant, ammonia is efficient and cost-effective. However, it is also toxic and corrosive, with a pungent odour that aids in leak detection.
Butane and isobutane
While both are flammable, butane is known for its use in cooking fuel, whereas isobutane serves as a refrigerant.
Carbon monoxide
A silent hazard. Highly toxic and can be explosive in certain concentrations.
Hydrogen
The most abundant element in the universe, hydrogen is a clean-burning gas with the potential for energy production.
Methane
The main component of natural gas, methane is odourless so must have an odour added to help detect leaks.
Propane
Propane is versatile, used in both vehicles and as a fuel source in remote areas, and is known for its ability to liquefy at moderate pressures.
Ethane
Mostly used to produce ethylene for plastics, ethane is also a potential feedstock for PVC and other materials.
Ethylene
A key gas in the ripening of fruits and manufacture of plastics and detergents, ethylene is also produced naturally by plants.
Silane
Used in semiconductor manufacturing, silane is pyrophoric and requires careful handling due to its spontaneous ignition in air.
Chlorine trifluoride
Although not flammable, this compound induces combustion in other substances and can only be stored in specific metal alloys due to its highly reactive nature.
Real-life implications and safety protocols

It is important to understand the conditions that can lead to industrial and household explosions. Methane leaks are common in households, but did you know that methane can accumulate underground naturally and cause significant risks? Another setting in which flammable gases require extra safety measures are spray painting facilities. In these explosive atmospheres, strict safety protocols are vital to prevent explosions.
The importance of SDSs
SDSs are critical in providing information on the handling of flammable gases, their hazards, and emergency measures. It’s a legal requirement to review these documents before working with any flammable substances.
The bottom line
Flammable gases are valuable in many areas, but they must be handled with care and knowledge. Staying alert and educated is key to using these gases safely and effectively.
Explore the complete article for more in-depth insights, proudly presented by Armadex, your trusted provider of explosion-proof devices for hazardous environments.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




