Making Fuel from Water
How electrolytic hydrogen can help power our future
The role of hydrogen
REDUCING greenhouse gas (GHG) emissions to avoid the catastrophic consequences of global warming is the most significant problem of our time. Hydrogen can play a key role in helping the UK achieve its GHG emission reduction targets. Major reductions of emissions have been achieved in the electricity sector by increasing renewable electricity penetration as well as phasing out coal-fired power plants, but as well as continuing to reduce emissions from electricity production, progress in the heating and transport sectors needs to be drastically improved. Hydrogen can play a major part in reducing emissions in these sectors, and if produced electrolytically, provides a link between them.
The majority of hydrogen produced currently is through steam methane reforming, and is then used in the chemical industry, for example for ammonia production. This method is not low carbon unless used in conjunction with carbon capture and storage (CCS). With CCS, a 60–85% reduction in GHG emissions is possible over methane.1 An alternative method to produce green hydrogen is via electrolysis. An electrolyser is a type of device that commonly used to decompose water using direct electric current. Hydrogen and oxygen will be produced from water through redox reactions. The overall reaction is:
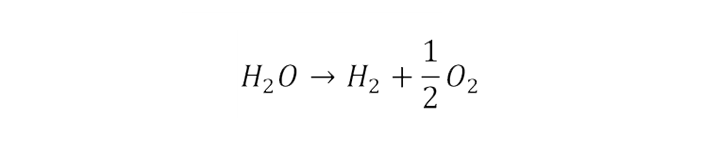
If the electricity used in the process is from low carbon sources, then the hydrogen is a low carbon fuel. The exact chemistry of the reaction depends on the particular technology used, with some technologies more suitable for certain applications.
Electrolyser technologies
The discovery of water electrolysis is disputed. One view argues that it was discovered by Anthony Carlisle and William Nicholson in 1800. The other view gives the credit to Johann Ritter. It is estimated that in 2017, the global sales of electrolysers reached 100 MW/y. This indicates a daily hydrogen production of 50,000 kg.2
There are three different types of electrolyte that can be used in a typical electrolysis process: a liquid electrolyte; a solid polymer electrolyte in the form of proton exchange membrane; or an oxygen ion conduction membrane. As a result, there are three main types of electrolysers, according to the adopted electrolyte technology, namely alkaline, proton exchange membrane (PEM), and solid oxide (SO) electrolysers.
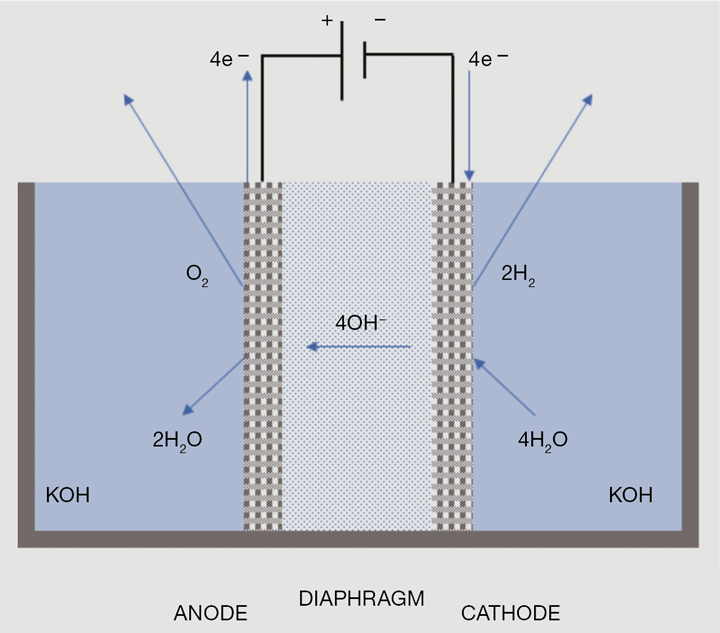
Alkaline electrolysers were the first commercialised electrolysis system and remain the most mature and widely used system for hydrogen production. Two electrodes, an anode and a cathode, often manufactured from nickel are immersed in a conductive electrolyte. A 30% aqueous solution of potassium hydroxide (KOH) is commonly used as the electrolyte to enhance ionic conductivity. However, sodium hydroxide (NaOH) can also be used. The separator is used to allow the migration of hydroxide anions (OH-) and avoid the mixing of hydrogen and oxygen gas generated. Despite the fact that it is an established technology, there are a number of technical disadvantages associated with alkaline electrolysers. This includes the use of corrosive electrolyte, low current density, limited turndown ratio and inability to operate at high pressures. A schematic is illustrated in Figure 1.
PEM electrolysers utilise a proton exchange membrane. The most commonly used membrane is Nafion, manufactured by DuPont with thicknesses ranging from 25–250 µm . Thicker membrane can be used to enable the electrolyser to cope with low load operation or frequent start-stops. This technology is at an early commercial stage. There is considerable ongoing research into the development of this technology.
Solid oxide electrolysers differ from both alkaline and PEM electrolysers in that instead of operating at about 80oC, they operate at a high temperature range of 800–1,000oC. At such high operation temperature, steam instead of water is fed into the electrolyser. The increased thermal demand is compensated by the decrease of electrical demand. When the thermal energy is provided as a waste stream from other high-temperature industrial processes, the cost for hydrogen production can be further reduced. However, it is identified that operating at such high temperature may cause a number of drawbacks, which includes poor long-term cell stability, interlayer diffusion, fabrication, and materials problems. There are research efforts in developing electrolysis cells working at intermediate temperatures (500–700oC). At the present time, SOE is still in the research, development and demonstration stage.
It is likely that the different technologies will be suitable for different applications. PEM based electrolysers are more able to quickly adjust their output and have an operating range of 0–100%, so are suited to applications such as demand-side management and renewables integration where flexibility of operation is a key demand. Alkaline electrolysers as a more established technology are likely to continue being used where rapidly variable input is not a key demand. Solid oxide electrolysers have the potential to offer highly efficient production of hydrogen if some of the heat used is provided by waste streams.
Applications
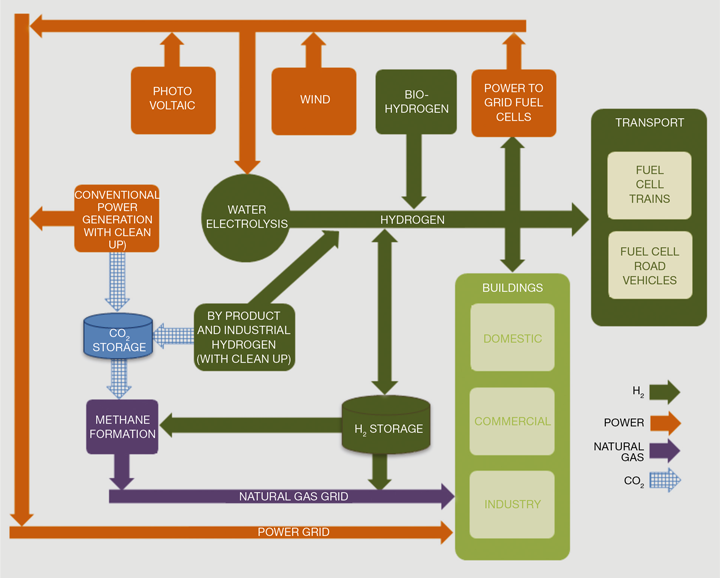
Hydrogen can be produced from a variety of sources such as fossil fuels, biomass, as well as electrolytically from water. It can be stored as a compressed gas, as a liquid, in solid state structures, or used to create synthetic fuels such as ammonia. Hydrogen can be used in fuel cells to generate electricity and heat, combusted, or used as a chemical feedstock amongst other methods. A large component of hydrogen produced in the future is likely to continue to come from fossil fuel sources if combined with CCS, due to the predicted hydrogen production costs from the different methods. However, due to its ability to support the operation of renewable electricity sources whilst providing a zero carbon fuel, electrolytically-produced hydrogen can play a key role across a number of different sectors. Hydrogen produced electrolytically is free from hydrocarbon contaminants, reducing the amount of cleanup required. This can increase the cost competitiveness of electrolytically-produced hydrogen, especially in applications requiring high purity hydrogen such as fuel cells. Figure 2 shows a schematic demonstrating how hydrogen can provide a link between the electricity, transport and gas sectors.
Renewables integration
Increasing amounts of renewable electricity production will necessitate the use of energy storage and demand-side management to alleviate constraints on the electricity network as well as allowing system wide balancing over different time scales. Hydrogen is particularly suitable to longer term energy storage, such as seasonal storage, due to its low energy storage related costs and can complement shorter term storage such as batteries. Hydrogen produced electrolytically can be compressed and stored, either in gas cylinders, or potentially in geological structures such as salt caverns. The stored hydrogen can later be used to produce electricity through a fuel cell as round trip storage.
Power to gas
As well as round trip energy storage, hydrogen can provide a link between the electricity and heating sectors by injecting electrolytically-produced hydrogen into the gas network. Current regulations severely restrict the percentage of hydrogen in the network, but investigations have shown that up to 8% hydrogen by energy is technically feasible without causing problems to consumers.1 If the hydrogen is produced from low carbon or renewable electricity, this has the ability to help decarbonise heating. Some schemes in the UK propose converting entire sections of the gas network to run on pure hydrogen. This hydrogen can then be used in converted gas boilers and other appliances, or be used in fuel cells to provide combined heat and power.
Vehicle fuelling (forecourt electrolysis)
Hydrogen fuel cell electric vehicles (HFCEVs) can play a key role in decarbonising transport emissions, particularly in heavier duty applications such as buses and goods vehicles in addition to large passenger cars and fleet vehicles. Hydrogen-related technologies can also contribute in decarbonising rail and shipping. The hydrogen can be produced electrolytically, either in large centralised facilities, or using forecourt electrolysers, providing a link between the electricity and transport sectors.
Industry
Industrial GHG emissions are difficult to reduce due to the need for high temperature heat in industrial processes, which is often provided by burning fossil fuels. As well as this, hydrogen used for chemical process reactions is currently mainly provided through steam methane reforming, with consequent GHG emissions. Production of hydrogen from steam methane reforming with CCS provides a route to partial decarbonisation of industry, which can be complemented and extended by using renewably-produced electrolytic hydrogen.
Case study – enabling hydrogen fuel cell electric vehicles
One of the issues with developing and introducing new technologies is the provision of infrastructure to enable early adopters of technology. A case in point is the use of hydrogen fuel cell electric vehicles (HFCEVs) which require development of a hydrogen fuelling infrastructure to precede the uptake of the vehicles.
Early adoption can be facilitated by commercial, public, and academic collaboration. An example is refuelling of HFCEVs operated by local organisations, such as the Wales and West Fire Service, which use the University of South Wales’ hydrogen refuelling facility located at the Baglan Hydrogen Centre, which was supported through such a collaboration. Figure 3 shows a HFCEV at the University of South Wales Baglan Hydrogen Centre.
The hydrogen generated at the Baglan site is partially produced through 20 kW of roof-top solar PV connected to an onsite alkaline electrolyser, with the balance supplied from the electricity network. The electrolyser can produce 10 Nm3/h of hydrogen, which is compressed and stored onsite. The hydrogen can then be used to produce power to the building or back to the electricity grid through an on-site fuel cell or be used to refuel vehicles. The vehicles can be refuelled in less than 5 minutes from the onsite storage, giving a potential maximum range of 594 km.3 With the size of electrolyser currently installed at the Baglan Hydrogen Centre, it takes about 5 hours to replenish the storage from a single vehicle fill.

Summary
Electrolytically-produced hydrogen has the capability to provide a GHG free fuel which can be used across multiple sectors and applications. It is likely that this will play a key role alongside other low GHG technologies in providing a route to decarbonisation of our future energy systems.
References
1. Hydrogen in a low-carbon economy. Committee on Climate Change. November 2018
3. https://www.hyundai.co.uk/about-us/environment/hydrogen-fuel-cell
The University of South Wales ‘Hydrogen Centre’ at Baglan, Port Talbot is an academic research and development centre which is part of the Sustainable Environment Research Centre at the University. Read more about the centre's work
This is the fifth article in a series discussing the challenges and opportunities of the hydrogen economy, developed in partnership with IChemE's Clean Energy Special Interest Group. For more entries visit the series hub.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




