Flow Batteries: Chemicals Operations that Promise Grid-Scale Storage
Adam Duckett speaks to flow battery innovators about the history of the technology and what’s to come
AS we plug more renewable sources of energy into our grids, engineers face a growing conundrum: how do we balance the increasing variability so that power is still available when we need it, even if the wind has dropped and clouds have drifted to a halt in front of the sun?
Ideally, we need a technology that can store renewable energy when it’s generated in excess and then quickly release it on demand so that we can stop relying on the gas- and coal-fired plants that are currently used to top up any troughs in our supplies.
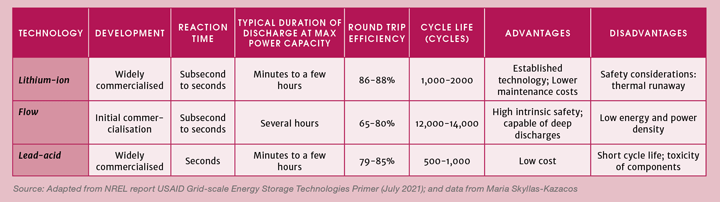
A technology receiving growing interest for grid-scale storage is flow batteries, whose proponents tout a list of benefits including long duration storage and inherently safer operations (see Table 1).
Propelled by oil shock
Flow batteries are not a new technology. In fact, their development began in earnest during the 1970s in the wake of the OPEC oil embargo when NASA was searching for ways to help shield the US from future energy shocks.
During this time, chemical engineer Robert Savinell, who is now an engineering professor at Case Western Reserve University in the US, was working with NASA during his PhD on the development of an iron-titanium system; though NASA would eventually pivot to focus on an iron-chromium system. But before we touch on the various chemistries available, how does a flow battery work?
First off, imagine a large-form factor system that is best suited to stationary applications. Flow batteries involve tanks filled with liquid electrolytes that are mechanically pumped through pipes to drive charge and discharge cycles. They have comparatively lower power and energy density so aren’t expected to find use in your electric car or mobile phone.
Among flow battery variants, redox is the most established. Savinell explains: “It’s an electrochemical device that allows you to take electrons in and store them by transforming chemicals from one [oxidation] state to another. And then you recover those electrons by having the chemicals go back to their original state.”
“The active materials are actually flowing through the battery and the electrodes are inert. It’s not like lithium batteries that take materials in and out of the electrodes. They’re just for transferring electrons.”
The energy and power capacity of redox flow batteries are separately scalable. We pick up the story with Maria Skyllas-Kazacos, an Australian chemical engineer and emeritus professor at the University of New South Wales (UNSW) who pioneered the development of the vanadium flow battery, which is the most established system in use today (see Figure 1).

“Flow batteries are really much more versatile than conventional batteries because they decouple the power and the energy capacity,” she says.
“Each cell gives you a certain voltage. The number of cells determines the total voltage and the size of your electrodes allows you to determine how much current you can pass through. So, current times voltage gives you the power. Whereas the energy capacity – the kilowatt hours – is dependent on the volume of your solutions.”
Increasing the storage capacity involves using a larger tank of electrolyte. By decoupling the systems, flow batteries are well placed to compete at higher storage capacities with lithium-ion batteries whose interlinked systems make their scale-up costs linear whereas those for flow plateau.
Despite its advantages, the flow battery has been relatively slow to find commercial application, though the pace is now picking up. In September, the world’s largest flow battery storage system – a 100 MW / 400 MWh vanadium system – was connected to the grid in Dalian, China. The Dalian Institute of Chemical Physics says there are plans to double the capacity of the plant.
NASA launchpad
Skyllas-Kazacos’ path to the flow battery and its subsequent commercialisation followed a convoluted path. She completed her undergraduate studies and a PhD on high temperature molten salts at UNSW in the 1970s. Keen to apply her expertise in electrochemistry, Skyllas-Kazacos went to Bell Telephone Laboratories in the US where she joined research efforts on liquid junction solar cells and lead-acid batteries.
On her return to UNSW, she led research on aluminium smelting but her experience at Bell Labs also saw her pulled back into the world of energy storage by a colleague working on lead-acid batteries. Then came her connection to flow batteries. UNSW’s Martin Green, who in February was awarded the QEPrize for Engineering for his foundational work on solar cell efficiency (p11), had become interested in NASA’s iron-chromium flow battery scheme. Given the electrical and chemical engineering crossover of the system, a masters student asked Skyllas-Kazacos if she would co-supervise the project.
“I wasn’t even aware of [the flow battery] until he introduced me to the work that NASA was doing.”
Skyllas-Kazacos says it quickly became evident that those early generations were flawed. The iron and chromium ions couldn’t be stopped from diffusing across the membrane separating the two half cells.
“After only a few dozen cycles, the iron and chromium would just mix and you would lose half of your capacity, which is very difficult to restore.”
A colleague who was working on mineral processing offered an intriguing solution.
“He said: ‘Vanadium has got a lot of oxidation states. Maybe you can have a look to see whether some of those might be suitable for a flow battery so you can have the same elements in both half cells.’”
He was proved right and Skyllas-Kazacos and her colleagues went on to discover that a defective electrode rather than a polished one aids the reversibility of the battery’s reactions; they identified sulfuric acid as the best electrolyte medium; and that the slow dissolution rate of vanadium-5 could be ignored by oxidising it from vanadium-4.
“By indirectly producing it we were able to make two molar solutions and then it became practical,” she says.
“I just thought we’d publish a few papers. I didn’t realise that 40 years later I’d still be working on vanadium batteries.”
Interest in the system took off in the late 1980s after the university published a newsletter profiling the work. Against the odds, the national media picked it up and turned the team’s early-stage research into front-page news. Then suddenly industry was knocking at the door. Licences and patents were sold to a vanadium miner who funded more research. But over the years, circumstances contrived to see the IP passed like a relay baton between companies and across continents.

A power-generating subsidiary of Mitsubishi Chemicals spotted an opportunity to use its waste streams of sulfur dioxide and soot laden with vanadium to manufacture flow batteries to store energy from their power stations. They built a 200 kW / 800 kWh demonstration plant but financial troubles came calling and the licenses and the technology they developed were sold to Sumitomo Electric Industries who conducted a number of large-scale demonstration projects up into the mid-2000s.
“They were able to prove the technology worked well for wind storage, for solar storage, for emergency back-up power, for load-levelling, and they were able to integrate it into all of the these different types of systems.”
And while the engineering had been proved, the system cost still wasn’t competitive, and the basic patents expired in 2006.
“Until about 2010, the market for large scale energy storage just wasn’t there,” Skyllas-Kazacos says. She was frustrated that some in the renewables industry were denying the need for energy storage to stabilise the grid.
Storm Musk
She says the turning point came in 2017 when Elon Musk said he’d solve South Australia’s blackout woes by installing a 100 MW lithium ion storage system within 100 days or provide the system for free.
“That attracted so much interest and everyone suddenly realised ‘Oh, actually, there are batteries around’.”
Skyllas-Kazacos says she’s now very optimistic about the future of flow batteries.
“Ten years ago, I was feeling quite frustrated,” she says, noting that lithium-ion had captured the imagination as the investment in gigafactories for electric vehicle batteries had helped to quickly reduce the costs.
“Vanadium and flow batteries just haven’t been receiving that sort of funding at all. But now people are realising that if we’re going to go with electric cars, should we be wasting all that lithium on building big batteries for solar and wind farms?”
Looking ahead
Skyllas-Kazacos says the longterm viability of flow batteries has been proved, pointing to a containerised Cellcube system in Germany that was taken offline after operating for ten years and tests showed it hadn’t lost any capacity or efficiency. Though there’s still much to do, and many contributions for chemical engineers to make.
“A flow battery is like a chemical process,” she says. “It doesn’t matter how good your battery is, if it’s not controlled and operated properly, it’s going to fail pretty quickly.”
She notes there is work underway to improve power density to shrink battery sizes and material costs; create improved membrane materials; and develop processes to produce electrolytes.
Skyllas-Kazacos’ face lights up as she discusses the partnership with North Harbour Clean Energy which last year committed to build a factory for vanadium batteries in Eastern Australia and install a 4 MW / 16 MWh system at the site of an unspecified industrial customer to showcase the technology.
It’s wonderful she says, after all these years, to have production coming to Australia.
“Why make everything in China?” she asks, echoing a common frustration that Australia exports its mineral wealth overseas when it could add more value at home.
Australia is estimated to have around 18% of the world’s vanadium resources. And while high prices have proved a sticking point, and one not helped by sudden fluctuations in demand from the steel industry, new business models that involve leasing vanadium for use in batteries will lower the costs to entry.
Other chemistries
While it’s the most developed, vanadium is not the only chemistry gaining interest. ESS, which is backed by BASF, has developed an iron-based flow battery system and went public in 2021.
Meanwhile, StorTera in the UK has been awarded £5m in government funding to build a containerised 200 kW / 1.6 MWh lithium sulfur system. The company has previously run two demonstration plants in Scotland and is now building a manufacturing facility in Edinburgh. CEO Gavin Park says the company is moving from a typical sandwich structure for its cells to a form factor that is easier to manufacture and improves power density.
“It has the potential to be one of the most energy dense battery technologies. So that has a lot of advantages in terms of its footprint and the applications it could be used for,” Park says.
“I think there will be a range of technologies that meet different needs for different use cases. There will be a lot of new energy storage technologies coming to market in the next decade.”
“Ultimately, it’s about enabling renewable energy. So we’re very much in it for the for the long term to try and do something meaningful.”
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




