Electrochemistry for greener steel

Amanda Jasi speaks to technology developers working to use electrolysis to reduce emissions from steel manufacture
STEEL is the most important engineering and construction material, and finds use in many areas, from infrastructure to vehicles and tools. But an analysis of IEA data suggests the steel sector is responsible for more than 10% of global CO2 emissions.
As the world strives to reduce damaging emissions, steel will continue to play a key role in society, making green steel production an important goal. It is a topic of focus for many companies, collaborations, and organisations.
The Chemical Engineer spoke to technology developers working to reduce emissions from the steel sector by using electrolysis to generate iron, that can then be alloyed to create steel. This would reduce the emissions directly from the manufacturing processes, as well as enable it to rely on renewable energy sources for further reductions.
From the tap
Daniel Bregante, pilot-scale program lead at Boston Metal, discussed the ongoing development of his company’s patented molten oxide electrolysis (MOE) technology for producing high value-metals. Bregante said that Boston Metal’s “big goal” is to decarbonise the metals industry, and within the hard-to-abate steel industry the “white whale” is decarbonisation of iron production. “Right now, we’re working on new technology to decarbonise primary iron-making,” he said.
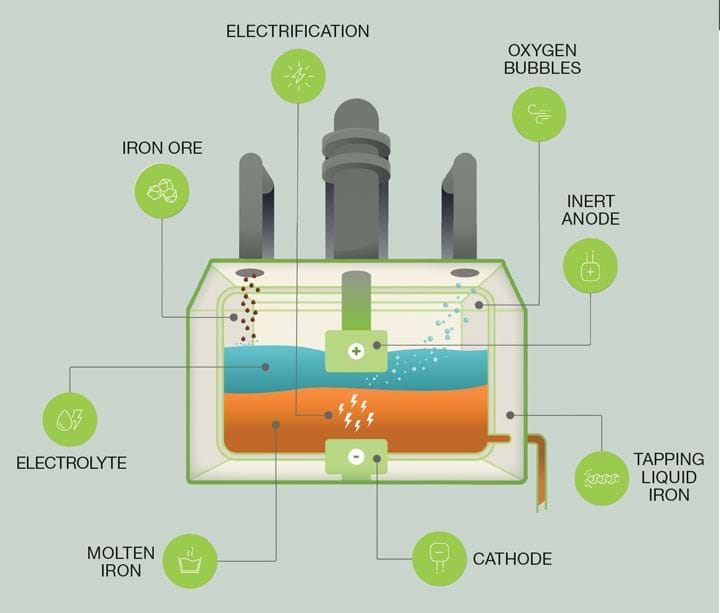
Boston Metal makes iron via bulk electrolysis. Extraction takes place in a large vat of electrolyte that is a mix of metal oxides, specifically containing iron oxide, with a proprietary metallic anode and an iron cathode. The cell is electrified and heated to 1,600°C for the process.
At the anode, at the top of the cell, an oxygen evolution reaction takes place, removing O2- from the vat of molten oxides and producing oxygen gas that leaves the cell. At the cathode, iron cations (Fe2+ and Fe3+) are reduced to produce molten iron metal (Fe0) that is incorporated into the cathode and forms a growing layer phase separated from the electrolyte due to differing density.
Bregante said that the process is semi-continuous, with iron production being achieved by passing electricity through the cell. Periodically, the cell is tapped to retrieve iron.
“You can imagine we have a literal spout at the side of the cell where we can then drill a small hole and actually drain out some of the iron. Then we plug that hole back up and we continue the process. We’re constantly making iron, depleting it, making iron, depleting it, so that the process can run for months or years on end.”

Boston Metal is currently scaling up its technology through a series of pilot scale cells, with Bregante coordinating the “inception” of cells and what goes into their design, and helping lead the research program associated with scaleup from lab to industrial cells. He said that right now the company has three different-sized pilot cells, the smallest of which are the lab cells operating at around 2–5 amps, producing about “10 or so grams of iron” per day. They also have a 150 amp pilot (1–2 kg/d) and a 2,500 amp cell (25–30 kg/d). The next pilot scale cell will operate at around 25,000 amps, producing “on the order of one ton per week”.
“For producing liquid iron, we have all of those scales except for the largest. Right now, our largest 25,000-amp cellsis working on ferroalloys, and that’s going to be retrofit in the next year to convert over to our steel decarbonisation technology.” Bregante explained that the cells can be modified to produce different metals by changing the components, the main difference being the constituents in the electrolyte.
Further down the line, by 2026, Bregante said he expects Boston Metal’s demonstrator will comprise five commercial-scale 200,000 amp modules with each module producing 3-4 t/d of iron. Over time, the company aims to increase the size of its modules to 600,000-amp and beyond, to reduce CAPEX and optimise for larger plants (>1m t/y).
Discussing some of the challenges involved as the company scales up, Bregante first acknowledged that a unique aspect of Boston Metal’s process it is self heating. The passage of thousands of amps of electricity between the anode and cathode, and through the molten oxide electrolyte in between, generates Joule heating within the system. “Essentially, we don’t have to have any external heat provided to the system to keep everything molten,” he said.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




