Digitalisation for Pharma
Tony Margetts explains the industry’s challenges around connecting and collecting
THE pharmaceutical industry is ripe for digitalisation, and chemical engineers have a key role to play in helping this conservative sector take advantage of its major benefits. It is a must for companies to stay competitive and compliant.
Digitalisation is the collection of process data; not to be confused with digitisation, which is the conversion of data into a digital format. It’s about setting up a compliant framework for collecting your data in an organised format. The organised data can be used in automatic data structuring and recording to better understand process variability; in tools for modelling or building digital representations of processes; or for understanding and reducing resources and time to bring life-saving medication to market.
Key requirements and benefits of digitalisation are:
- shop-floor improvements to process performance, product quality and safety;
- providing electronic work instructions at point of use;
- collecting data from point of use and producing reports;
- increased right-first-time by reporting defects at the source with the ability to communicate and decide on the shop floor at the point of use;
- standardisation of manual processes;
- faster training times and recorded training;
- real-time reporting on critical process parameters (CPPs) and key performance indicators (KPIs) leading to higher throughput and better quality; and
- enforced compliance with the regulations, especially data integrity and ALCOA (attributable, legible, contemporaneous, original and accurate).
The challenges for pharma
Digitalisation provides continuous monitoring of critical parameters which improves product quality and patient safety. The problem for pharma is that current systems don’t provide continuous monitoring. While we have DCS and other process control systems, many pharma operations are manual, which makes collecting data difficult.
Continuous monitoring also allows continuous process verification, which simplifies the validation process, an important component of pharmaceutical manufacture. It establishes that the quality attributes and process parameters important for product quality can be met consistently. Continuous process verification can be used as an alternative to traditional process validation when it has been scientifically established during development that the control strategy provides a high degree of assurance of product quality1.
What is ICH Q10 and why is it important?
ICH, the International Conference on Harmonisation (www.ich.org) is a collaboration of medicine regulatory agencies which produces technical guidelines and requirements for medicinal product registration worldwide. It also facilitates adoption of new or improved technical research and development approaches which update or replace current practices in the pharma industry.
It has developed a set of guiding principles, the Pharmaceutical Quality System (ICH Q10)2, based on International Organization for Standardization (ISO) quality concepts and applicable good manufacturing practice (GMP) regulations. It applies to development and manufacture of pharmaceutical drug substances and drug products, including biotechnology and biological products, throughout the product lifecycle.
ICH Q10 includes the concepts: process performance and product quality monitoring system; corrective action and preventive action (CAPA) system; change management system; and management review of process.
So how does digitalisation relate to ICH Q10? Data collected through digitalisation can be used to enable or improve processes by leveraging digital technologies3.
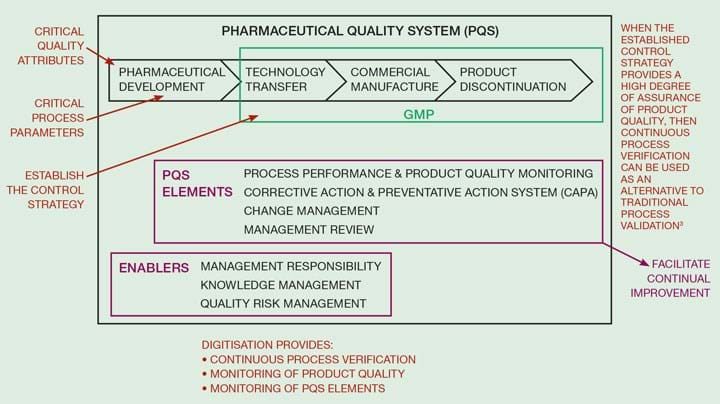
Digitalisation in pharma uses quality by design (QbD) principles based on the ICH Q10 concepts. This provides assurance of the continued suitability and capability of processes. Figure 1 shows how digitalisation can be used as a means to implement ICH Q10. It refers to critical quality attributes, critical process parameters, control strategy, and the need to facilitate continuous verification.
Critical quality attributes (CQAs) are the attributes of the medicine which apply to any “physical, chemical, biological, or microbiological property or characteristic” that must be within a limit or range to ensure the pharmaceutical product meets the required quality standard (US FDA definition). Examples for tablets include strength, purity, content uniformity, and dissolution.
A critical process parameter (CPP) is a process variable that can impact CQAs. Therefore, CPPs must be monitored to enable early and accurate detection of deviations outside acceptable limits that will impact product quality. Examples for tablets include weights dispensed, particle size, blending time and speed, and tablet compression speed and force.
ICH Q10 covers the pharmaceutical quality system which includes the product life cycle and the GMP parts shown in Figure 1. CQAs and CPPs are developed and identified during process development. Risk management is used to link the CPPs to the CQAs and to develop the control strategy. Using digitalisation to develop the control strategy allows the concept of continuous process verification by using the digital measurements to continuously check the product quality.
What about Industry 4.0?
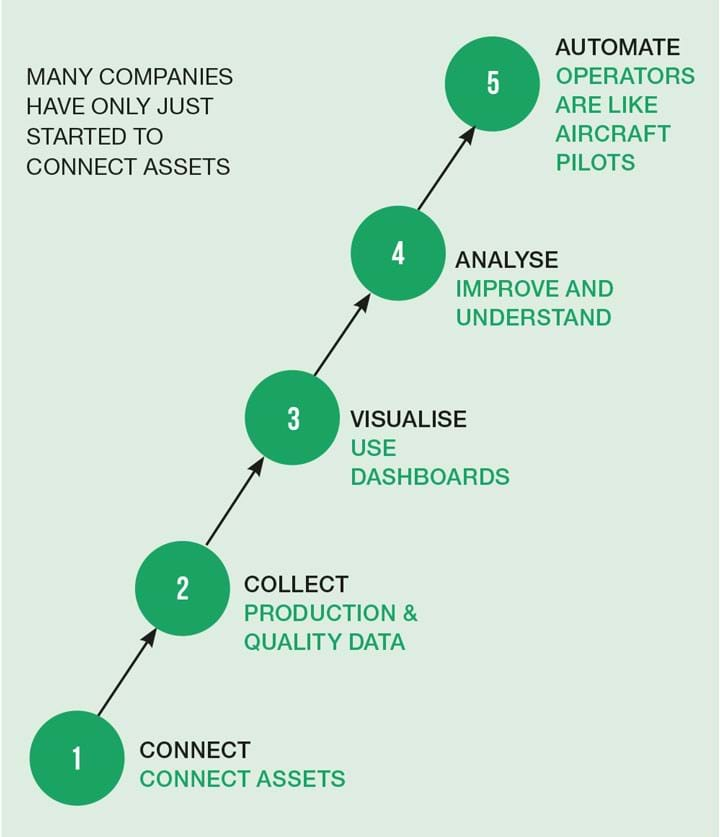
Digitalisation is part of Industry 4.0, as described in Figure 2. As more data is available it is possible to analyse in depth, for example by using machine learning. When enough data has been collected and analysed it is possible to automate the process. In pharma, a major problem is collecting data from the many different systems involved and the large number of human actions involved, and to be able to continuously monitor the process performance and product quality.
Let’s look further at some aspects of the connect and collect steps in Figure 2.
Challenge of different computer systems
The pharma industry has to collect a lot of data and records to be able to sell the product, via many different computer systems, which typically do not talk to each other.
For example, the supply chain might be managed using SAP (system applications and products), while the activated pharmaceutical ingredient (API) process is typically controlled using a distributed control system (DCS) or programmable logic control (PLC)-based system. At the same time, the formulation process consists of various machines which have their own control systems (which are not connected), and the lab has many different types of instruments, many of which have a standalone computer to manage the instrument and collect the data.
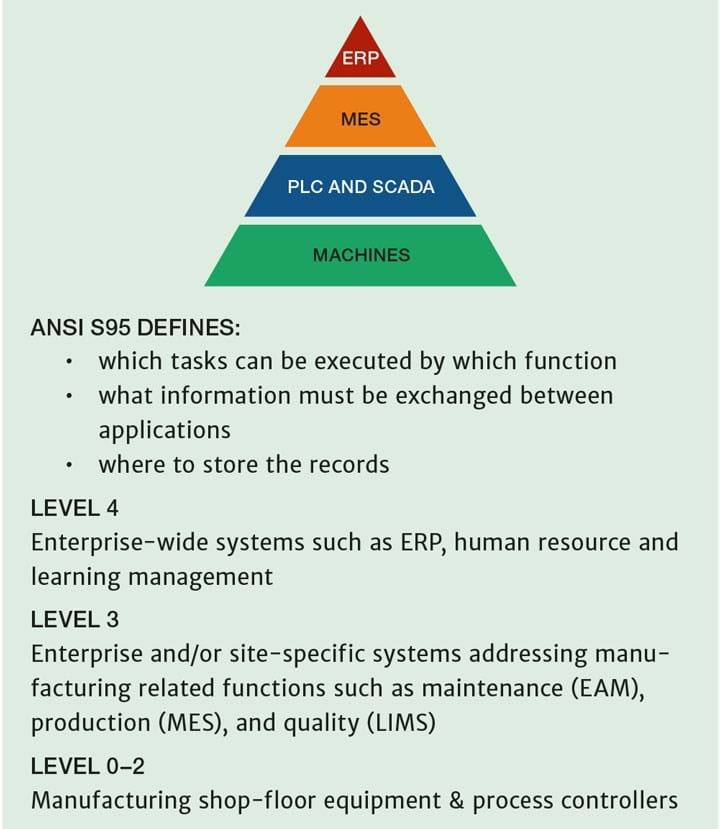
Traditional factory systems (see Figure 3) are based on ANSI/ISA 95 concepts4. The business is run with enterprise resource planning (ERP); the shop floor with a manufacturing execution system (MES); the machines with PLCs and supervisory control and data acquisition (SCADA) systems. Connecting all of these is challenging and expensive and may be acceptable for continuous processes, but often does not work for batch processes, which are common in pharma. Also, it is not so easy to manage data collection related to the human actions required which need to fit in between these systems.
How best to connect and collect for pharma?
Going back to Figure 2, the first steps are to connect and collect from inputs on the shop floor. Some of these are from human actions and some from automated processes. Examples include operator identification, room cleaning, equipment preparation, checking weight dispensing, checking the correct operation of the equipment by monitoring CPPs, normal operation and alarm conditions, water monitoring, environmental monitoring, microbial monitoring, process deviations, and many other types of data.
Top-down and bottom-up
Companies need to set some direction from top down and create a framework to collect data in an organised format so that it can be processed and analysed for process or safety improvement. What data is important? How should it be selected, collected and stored? The risk assessment process is used to make these decisions. The data must be labelled in order for the product, the batch, the equipment, the critical parameters, time and date, the operator, and other key meta data to be identified.
A flexible bottom-up approach is required to collect data from a wide range of different systems. We need to think about collecting the data from a bottom-up Internet of Things (IoT) connectivity view rather than the traditional top-down hierarchy of S95.
We need to collect key data from human actions. In pharma and biotech processes there are many manual operations that are required to fill the gaps between the existing system solutions. Capturing data from these operations at the point of use and ensuring right-first-time operation is critical. I know a tragic example of a faulty line clearance, which resulted in a rogue label being attached to a vial; a pregnant woman subsequently received an injection which killed her and her unborn baby.
Regulations
Data collected by applications running in the healthcare industries has to comply with the regulations, particularly data integrity5. Data has to be: attributable; legible; contemporaneous (recorded at the time the data is created); original; and accurate.
These requirements are sometimes called ALCOA requirements and the recorded data has to include this important meta-data and other associated data such as the date and time, and all of this is stored with the primary data.
Manufacturing IT solutions
Apps are now available which provide a configurable manufacturing platform that connects people, sensors and machines to provide the connect-and-collect requirements described above.
These web-based software tools (available on tablet computers or even smartphones) are easily and rapidly configured to model process steps and sequences without using code and without the need for large and costly IT systems.
They can be used on the shop floor to help the operator and user interact to record data that allows greater visibility of the operation to other operators and managers. Issues and errors can be dealt with immediately and notifications are built in using email or phone messaging for automating communication around the factory.
There are a number of low- or no-code platforms available for building apps, but the important requirements are the ability to connect to a wide range of input devices and other databases and to manage the data in compliance with regulations. Examples include: Redzone digital production system6, GainSeeker7, and Tulip8.
Cases
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




