A Life in Lithium
The future of energy needs chemical engineers, and lots of them, say Jacob Brown, Titi Oliyide, Laurent Petithuguenin, and James Sweeney
IT IS now a year since Tesla’s “Battery Day” announcements and it seems that rarely a week goes by without a headline on some kind of new lithium-ion battery (LIB) development. Whether for electric vehicles (EVs), or the storage of wind- and solar-generated electricity, LIBs are increasingly finding a way into our energy-driven lifestyles. As our collective need to decarbonise becomes clearer, serious implications are emerging; the energy industry is a megatonne industry, and enormous quantities of LIBs will need to be manufactured on a daily basis.
LIB production volumes are already sizeable, with current estimates in the range of a few million tonnes per year – equivalent to 130-250 GWh/y. In just 10 years, this number is expected to increase tenfold to 1,300 GWh/y. Even with such aggressive manufacturing ramps, the number is still not high enough. To decarbonise the current global, fossil-powered car fleet, providing every car with an 85 kWh battery pack, we would have to produce the order of 80,000 GWh of battery cells. The story told by the numbers is clear: to build a new energy ecosystem inclusive of battery electric vehicles, an enormous influx of capital, resources and human talent is needed for the LIB industry.
Batteries are supposedly going to be everywhere, but the question is, who will build them?
For targets to be reached, there needs to be a mass mobilisation of engineers into the LIB industry. The expertise of many engineering disciplines is required, and chemical engineers stand particularly well-placed. Much like the pharmaceuticals, catalysis, and semiconductor industries, the LIB industry produces a complex chemical product. This requires a strong understanding of physical and chemical fundamentals, as well as skillsets in process optimisation and quality assurance. So, why don’t we all have a friend in battery engineering? It would seem like so many engineers are needed that surely some of our colleagues might have made the jump. However, the industry is new, and there are not yet pipelines to recruit talent fast enough for the scaleup that the industry requires.
This article aims to showcase the component parts of the LIB supply chain so that we might reduce the barriers to entry for interested chemical engineers. As recruiters scour the world for battery talent, we want to help aspiring battery engineers to get involved and join the effort.
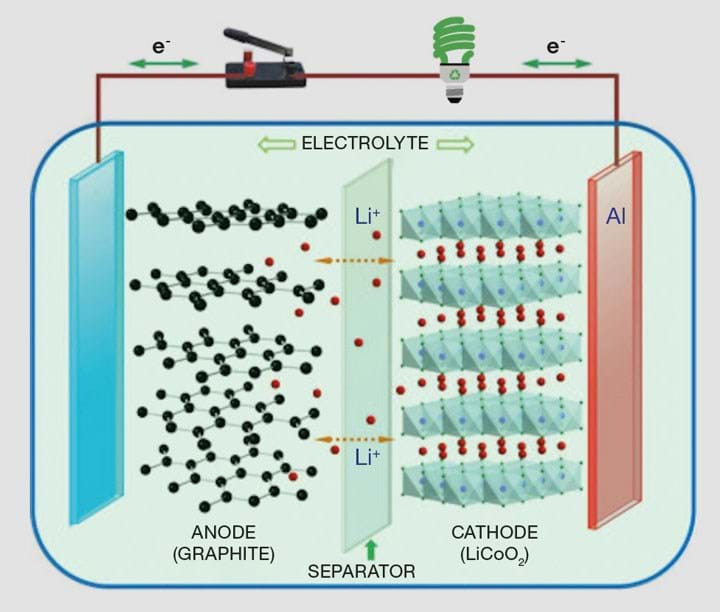
What’s in a lithium-ion battery?
Before discussing the manufacturing process, it’s helpful to explain some of the components of an LIB. A schematic is shown in Figure 1.
Fundamentally, LIBs consist of chemically-active materials that accept electrons upon reaction with lithium ions. These materials, when combined with conductive additives, become the device’s electrodes. Two electrodes are used in LIB cells – one providing a high energy “home” for the lithium (the anode), the other providing a low energy “home” (the cathode). As the cell discharges, lithium reacts out of the high energy home, ionising into the electrolyte and producing high-voltage electrons. Metallic foils then carry these electrons out of the cell to the cathode where lithium ions react into the material structure, receiving the electrons that were originally given off. As the lithium in the cathode has a lower enthalpy than the lithium in the anode, this whole process gives off energy, manifested as a voltage in the electrons flowing through the circuit.
The “trick” to a lithium-ion battery is in the nature of the materials themselves. Not only must they be able to react reversibly with lithium ions hundreds of times, but they must also be able to intercalate into the material, without damaging the physical or structural properties of the material. The materials used in an LIB are not uncommon on Earth, though they must be carefully processed to ensure material longevity and performance. Cathodes are typically comprised of metal-oxide or phosphate materials (eg nickel/cobalt oxide or iron phosphate) and the anode materials are made of graphite or silicon. An electrolyte composed of organic carbonates and a conductivity-enhancing salt facilitates lithium transfer from one electrode to the other and allows the reaction to be completed. Despite the name, there is no pure metallic lithium within current generation LIBs; it is only present in ionic form bonded to other materials.
LIBs are complex chemical devices, but the synthesis of their components is nothing new. Similar expertise and processing know-how has been demonstrated for decades in a variety of other chemical engineering industries – that knowledge and experience is now needed to improve LIB manufacturing processes. This is where chemical engineers can – and should – come in.
Jobs in LIB manufacturing
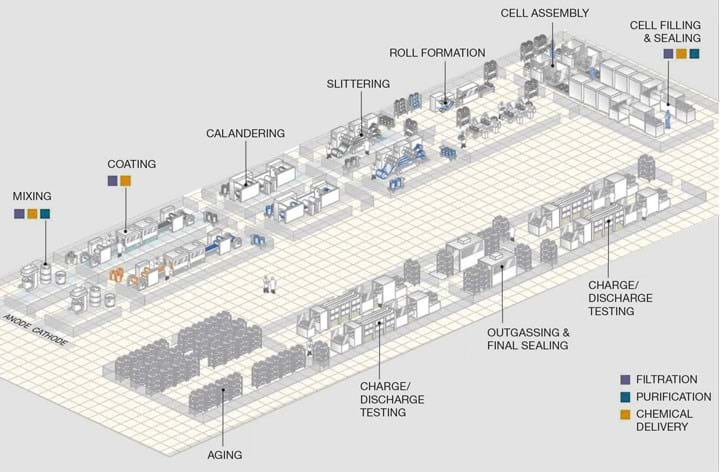
The LIB industry is fast evolving, and there are already several robust areas for chemical engineers to get involved. Whilst a large amount of LIB making is an “assembly” operation, the production of the electrodes is almost entirely a chemical process. In such operations, chemical engineering activities such as slurry-handling, mixing, coating, and drying are all of paramount importance.
At a high level, the LIB cell production process can be broken down into three main process steps: electrode manufacturing, assembly of the cell, and cell finishing. Each step is discussed below.
1. Electrode manufacturing: Of significant relevance to chemical engineers, the start of the LIB manufacturing process typically involves the fabrication of the device’s electrodes.
- The powder-based electrode components (active material, carbon black, and polymer binder) are blended in mixing vessels with an organic or aqueous solvent to disperse the components and dissolve the polymer binder. The three electrode components each have separate roles to play: the active material reacts with the lithium, carbon black is used to provide electronic conductivity, and the polymer binder sticks it all together.
Critical quality assurance considerations (QACs): component ratios and particle size, % solids, mixing time - The produced slurry is conditioned by continuous mixing until appropriate viscosities and flowabilities are obtained. This is often performed under vacuum to prevent air entrainment.
QACs: shear rate, viscosity, segregation of components, powder agglomerates - The slurry is fed through a slot-die (or similar device) to make a uniform coating (typically, a few hundred microns thick) on a large sheet of metallic foil.
QACs: film thickness, uniformity (to avoid streaks), over-coating - The wet, coated foil is fed through a drying oven to evaporate the solvent, precipitating the polymer, and binding the whole electrode together.
QACs: drying rate, uniformity (to avoid bubbling “volcanoes”), component segregation, film cracking - The dried electrode is then calendered through a large set of compressive metal rollers to increase electrode density.
QACs: compressive pressure, film density, particle cracking, film warping, film cracking - Cutting of the electrode into smaller sections and subsequent vacuum drying to remove residual moisture, solvents and atmospheric contaminants.
QACs: residual moisture, particulate contamination control
2. Cell assembly: Alternating anode and cathode electrode sheets are stacked on top of each other with plastic separators inserted in between. The cell is then packaged and filled with electrolyte.
3. Cell finishing: This is an unusual, electrochemical process step unique to LIB manufacturing. The completed battery cell is charged for the first time, encouraging a pseudo-polymerisation reaction of the electrolyte onto the active electrode materials. This process creates a passivating layer on the electrode surface and protects the materials from further degradation.
As seen above, the LIB manufacturing contains many opportunities for chemical engineering development. Most of the process steps have historical precedent in other industries, particularly where slurries, powders and films play essential roles. As LIB manufacturing scales up, chemical engineers will play a leading role in introducing expertise from other industrial settings.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




