New integrated, net-negative system captures carbon and produces ethylene
FOCUSED on integrating technologies that capture and use CO2 in a closed loop carbon cycle, engineers at the University of Illinois Chicago (UIC), US have built a small-scale machine that captures CO2 from flue gas and converts it to ethylene for use in industry.
Ethylene, with a worldwide consumption of around 200m t/y, is used in products as varied as antifreeze, medical sterilisers, polyethylene for plastics, and other organics. But to produce it via the conventional method means steam cracking ethane (C2H6) in a chemical plant, a process that uses extreme temperatures and pressures and releases substantial amounts of CO2.
Finding ways to decarbonise prolific CO2 emitting industries such as these has been an area of research for some time, but team leader Meenesh Singh says that the method used in their studies, is the first to achieve nearly 100% utilisation of CO2 to produce hydrocarbons. And, not only does the system run on electricity, but it also removes more carbon from the environment than it generates – a process dubbed net-negative.
“This is the first demonstration of a net-negative, all-electric integrated system to capture carbon from pollutants and create a highly valuable resource,” said Singh, an assistant professor in the department of chemical engineering at UIC. “Currently, integrated carbon capture and conversion systems are highly energy-intensive and work in a discontinuous cycle of carbon dioxide capture and reduction. Efficiently integrating carbon capture with the conversion system eliminates the need for transportation and storage, and thereby increasing its energy efficiency.”
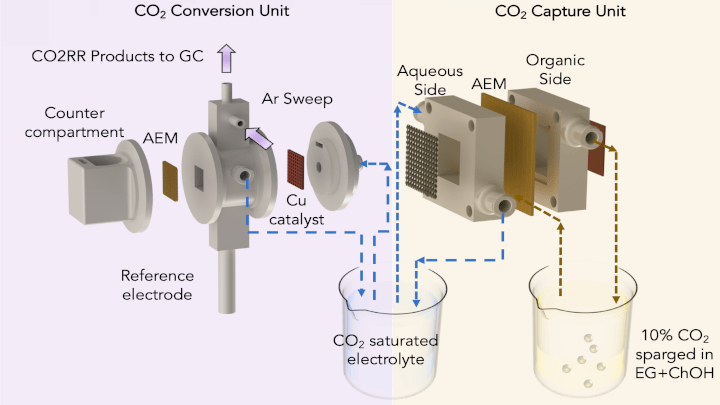
The team’s approach to carbon capture involves a modified standard artificial leaf system which includes a water gradient — a dry side and a wet side — across an electrically charged membrane.
It works by capturing CO2 in a CO2-binding organic liquid to produce a concentration of bicarbonate, or baking soda, on the membrane’s dry side. Negatively charged ions are then pulled across the membrane towards a positively charged electrode in an aqueous solution on the membrane’s wet side. The liquid solution dissolves the bicarbonate and converts it into CO2, that can be released and harnessed for CO2 conversion.
To convert the captured CO2 to ethylene, the team used a second system in which an electric current is passed through a cell containing the captured CO2 on one side, and a water-based solution on the other. Charged hydrogen atoms from dissociated water molecules are drawn into the other half of the unit, where they combine with charged carbon atoms to form ethylene.
The two systems are integrated by feeding the captured CO2 solution to the carbon conversion system and recycling it back. “The simulated flue gas sparged at the organic side of the capture unit was converted to HCO3- and transported to the aqueous side across the AEM as dissolved CO2, HCO3-, and CO32-. The CO2RR [CO2 reduction reaction] unit then utilized the dissolved CO2 and converted it to value-added products and fuels such as CO, HCOOH, CH4, C2H4, C2H5OH, and C3H7OH,” the authors explain in their research paper.
This method ensures a constant supply of CO2 from flue gas to produce high-purity ethylene. In addition, the system uses a modular, stackable design that allows the system to be easily scaled up and down.
So far, the team, who have received support from the US Department of Energy and Braskem, the largest petrochemical company in Latin America, have captured carbon at a rate of 24 g/d and produced ethylene at a rate of 188 mg/d.
Their next step is to scale up the system to produce ethylene at a rate of 1 kg/d and capture carbon at a rate higher than “kilograms per day", said Singh. "In the journey to make ethylene production green, this is a potential breakthrough," Singh added.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




