Tangled up in Blue

Tom Baxter argues against using blue hydrogen to produce electricity
EQUINOR's plans to provide "blue" hydrogen to the UK's Keadby Power Station in North Lincolnshire has received recent press coverage. Yet the motivation for using blue hydrogen to produce electricity is lost on me. It will result in more emissions, it will cost more and is less inherently safe than a conventional direct gas-fired power station with carbon capture and storage (CCS).
Blue hydrogen is produced by steam methane reforming (SMR) with a bolt-on CCS facility. The process produces carbon dioxide and hydrogen, with additional CO2 being produced from firing the reformer. Typically 9–10 t of CO2 results per tonne of synthesised hydrogen. The CO2 is captured and compressed for transport to a geological trap. The hydrogen is combusted in a power station to produce electricity.
Producing electricity with the same low carbon footprint as blue hydrogen can be achieved using a conventional natural gas-fired power station with a bolt-on CCS facility.
The two routes, blue hydrogen and natural gas with CCS, are shown in the figure.
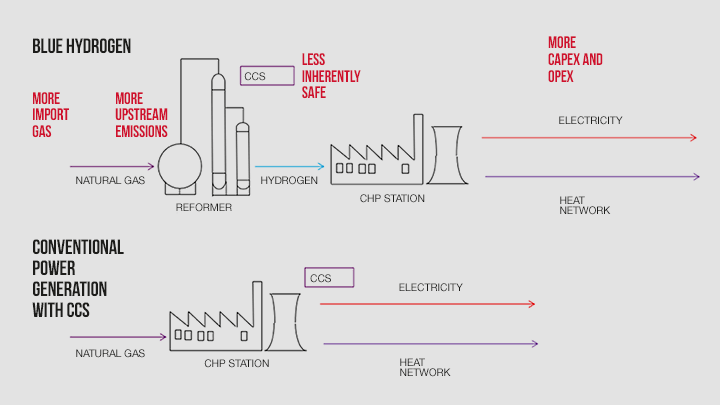
Comparing the two processes, the following can be concluded.
Blue hydrogen introduces the energy-intensive SMR step in the route to electricity. That results in more natural gas being used – approximately 30% – than the direct natural gas power station route. The upshot is more imported UK gas with blue hydrogen and more emissions from the gas producers.
The SMR plant is an additional cost in the route to low-carbon electricity. Furthermore, operating and maintenance costs will be higher with blue hydrogen.
Inherent safety is always a key discriminator when faced with a choice of options. The principles of inherent safety are:
- Eliminate – design to remove the need for hazardous material.
- Prevent – design to make the hazard less likely to occur.
- Control - design to control hazard within design envelope.
- Mitigate – design to reduce magnitude of hazard if realised.
On all of the above criteria, the introduction of the SMR plant makes the blue hydrogen route less inherently safe compared to direct natural gas power generation with CCS.
So blue hydrogen results in more import gas and more emissions. It costs more to build and operate and it is less inherently safe. Taxpayers’ money is being used for the Keadby proposal – why?
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




