Rules of Thumb: Flammable Liquids: Open or Closed?
Stephen Hall discusses the golden rules for design
IT is somewhat surprising how frequently this question is raised and how confusion reigns when answering it: Is a process open to the environment, and why does it matter?
I am discussing the use of the “open or closed” concept for flammable liquids in this article. Fire codes, such as the International Code Council’s International Fire Code (IFC), distinguish between open or closed systems when describing the use of flammable chemicals. This is important because vapours emitted from an open system can possibly catch fire, and flammable liquids in an open system are more likely to feed and prolong a fire. The codes specify requirements for certain aspects of the building and process equipment design under circumstances where flammable liquids are present. Sound engineering judgement is necessary, however, to determine if the system presents the hazards that the code addresses. This is where the question of open or closed processing fits in.
“Open” means that the flammable liquid is in direct contact with its surrounding environment, the space that is outside of the container that holds the liquid. But conditions that I will describe influence the degree of hazard that this contact presents.

Flammable liquids and flash point
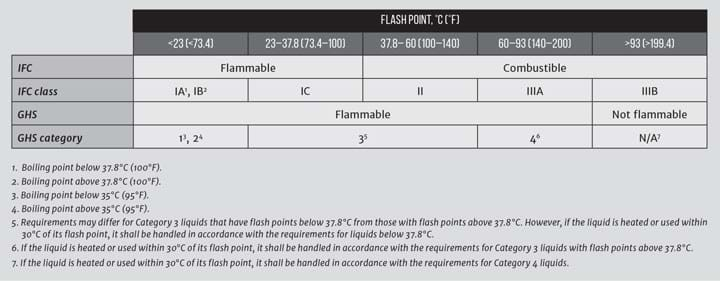
Flash points are useful for determining the need for spark-proof electrical classifications and for deciding how fire resistant a building structure should be. However, the flammable liquids do not present a credible risk for starting a fire unless they are exposed to the surrounding area. A liquid that flows inside a fully-welded pipeline poses no threat unless there is a leak or if the pipe is broken. The codes recognise this by distinguishing between “open” and “closed” operations. Before getting into that, I will review the science behind a flash point and discuss practical nuances that cloud the definitions of “open” and “closed.”
When a liquid ignites, the combustion is occurring in the vapour-air mixture that is at or above the liquid’s surface. The vapour concentration must be in a certain ratio to oxygen (from the air) to start and maintain the fire. The minimum concentration (by volume) in air is called the lower flammability limit (LFL) or lower explosive limit (LEL). Similarly, the maximum concentration is the upper flammability limit (UFL), or upper explosive limit (UEL).
From thermodynamics, the concentration is described by vapour-liquid equilibrium (VLE). In a closed vessel, the vapour pressure of an ideal liquid, Pi*, multiplied by the molar concentration in the liquid, xi, is the partial pressure in the vapour space. Divide xiPi* by the total pressure, P, to find the vapour-phase concentration, yi. This is Raoult’s Law. Because vapour pressure is a function of temperature, the flash point is the temperature that gives a vapour concentration that is equal to the LEL.
Substantial safety factors are used when designing safety systems. Alarms are typically set to trigger if the vapour concentration reaches 25% of the LEL; liquids are treated as being flammable if they are brought within 30°C of their flash points
VLE is a local phenomenon at the liquid surface. But due to diffusion in the vapour phase, the concentration throughout a closed space will equilibrate. If there is no bound to the vapour – that is if the liquid is open to the room – then the vapour will spread throughout. There will be a gradient in concentration that reduces with increased distance from the liquid. The slope of the gradient, and the concentration uniformity at points that are equidistant from the surface, are affected by the vapour density and ventilation rate.
Because the vapours of different compounds occupy equimolar volumes, and there is usually very little intermolecular interaction, compounds with a molecular mass less than 29 will tend to rise (eg hydrogen, natural gas), and those with a greater mass will tend to sink (eg most other flammable compounds). In a room that has unventilated pits or trenches, if hydrocarbon vapours are emitted, they may accumulate in a scale pit, trench drain, or sump and reach a concentration that exceeds the LEL even if the liquid is maintained at a temperature that is below its flash point. This is one of the reasons that substantial safety factors are used when designing safety systems. Alarms are typically set to trigger if the vapour concentration reaches 25% of the LEL; liquids are treated as being flammable if they are brought within 30°C of their flash points.
Addressing the fire hazard
The codes define flammable liquids in terms of their flash points. This is a convenience that helps categorise the fluids into hazard classes. The Globally Harmonised System (GHS) of Classification and Labelling of Chemicals is an internationally agreed-upon standard that is managed by the United Nations; it replaces standards that local authorities used previously (see Table 1).
Whenever flammable liquids are stored and used in a facility, a risk assessment should be performed to help identify and evaluate the hazards that the liquids may pose. From the foundation that I just outlined, these are some guidelines to consider:
- Applicable building and fire codes take precedence over any analysis or justification that you provide. However, local code officials may be empowered to override the code requirements. I do not recommend seeking an override; even if your analysis and justification are solid, it is risky to rely on the charity of an individual who could be replaced or have a change of heart with the determination reversed in the future.
- The quantity of liquid being stored or used in any one container, along with the surface area of the liquid in the container, determine how much vapour can enter the environment to cause a hazard. A spilled container that covers the entire floor of a room to a depth of several centimetres is clearly more dangerous than spilling a half litre that can quickly be cleaned up with a towel.
- The temperature of the liquid is important. Although the vapour concentration at the surface of a liquid that is held near its flash point temperature is sufficient to ignite if an ignition source is present, it is unlikely to ignite from a spark that is generated a short distance away from the surface. On the other hand, if the temperature gives a concentration that is higher than the midpoint between the LEL and UEL, you could expect an explosive concentration to exist at a significant distance from the source.
- The rate of ventilation determines the distance that an excessive vapour concentration extends from the liquid source. The codes define this in terms of air changes in the room and generally agree that six air changes per hour are enough to dissipate the vapours. The reliability of the ventilation must be considered.
- Where hazardous vapours will be emitted, evaluate any high and low cavities in the room or area. Even outdoors, a pit, such as a spill control dike for a tank, can accumulate an excessive concentration of vapours.
Relationship to codes
The IFC dictates that facilities be categorised into occupancy classifications such as office, factory, or warehouse. Each occupancy carries certain rules for fire resistance and protection. When a facility has more than one occupancy, the IFC specifies the fire resistance that is required between them.
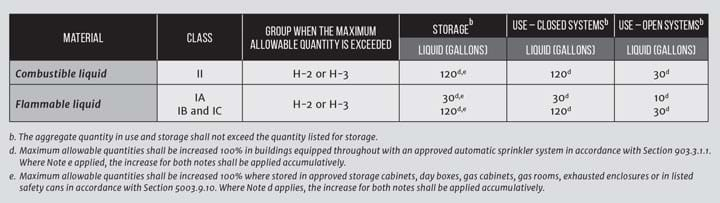
The IFC also grants leeway to store and use certain quantities of flammable liquids in any occupancy. If the quantities listed in the IFC tables might be exceeded, the occupancy must be classified as high hazard, or H. This triggers additional measures that address fire resistance and personnel safety, such as travel distances to building exits.
The IFC tables distinguish between open and closed use. The tables provide a much higher allowance for flammable liquids than in closed containers compared with the allowance for open use (see Table 2). Additional provisions that are applicable to open systems are described elsewhere in the code.
What is an Open System?
A flammable liquid in a vessel that has no cover obviously is open to the surrounding environment. The vapour-liquid equilibria concepts in this article apply. The fire codes recommend, in this case, the electrical classification within a certain distance (eg 1 m) be explosion-proof (Zone 0 or 1) and a buffer area beyond that (eg another 1 m) be electrically classified as Zone 2. The codes recognise that the nature of the environment is important; in an enclosed space that has very little ventilation, the entire room should be classified as Zone 0, whereas an outdoor or powerfully-ventilated area may have reduced classifications.
When the flammable liquid is completely contained in a vessel or pipeline with no vents to the surrounding space, it is closed. In this case, there is no possibility short of a catastrophic rupture that vapours from the liquid will enter the environment. The electrical classification is “ordinary,” and the quantities in Table 2 for closed storage and use are applicable.
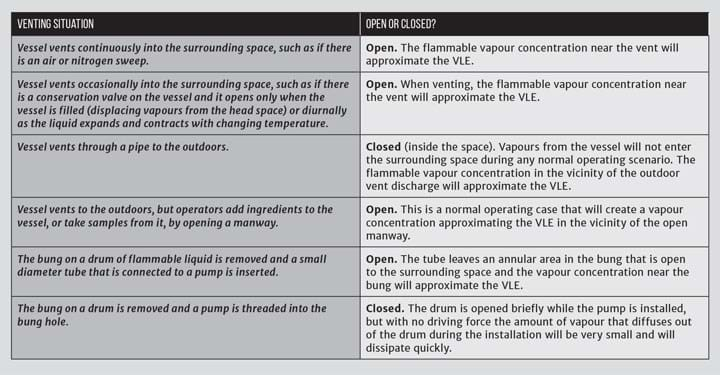
Questions arise when the vessel is vented into the space. Table 3 gives my opinions and rationale for various venting situations. Bear in mind that other scenarios may mimic the venting descriptions. For example, an agitated vessel that is vented outdoors but with a continuously-leaking seal may be considered open. Similarly, a vessel that is vented outdoors but equipped with a pressure relief valve that discharges inside the space may be considered open.
Conclusions
The characterisation of a system that stores and uses flammable liquids is important from a regulatory (ie code) perspective and is critical for determining safeguards that will prevent or mitigate fires and explosions. This article presents the theory that underlies that characterisation. But each situation should be evaluated on its own merits.
The grey areas may be difficult to discern and problematic to characterise... Therefore, it is important to use sound engineering judgement in determining whether a system is open or closed
Hazardous concentrations of vapours do not appear instantly when a flammable liquid is opened to the environment. But given time and in the absence of ventilation, the concentration will equalise according to chemical engineering VLE principles. This means that low points such as trenches and pits can harbour an explosive concentration of vapours even if the source is a very slow drip from a pump seal or leaking valve.
The fire codes were developed in response to real-world incidents and disasters. They are, in general, very conservative. They are also practical in the sense that the code writers recognise realities. The 120-gallon (454 L) threshold for Class IIB flammable liquids listed in Table 2 is not arbitrary: it evolved from the fact that liquids are commonly shipped in 55 gallon drums. Two drums neatly fit within the 120 gallon limit.
Did I answer the question? Although in most situations it is clear whether a system is open or closed, the grey areas may be difficult to discern and problematic to characterise. So no, I did not provide the definitive yes or no that everyone seeks. Therefore, it is important to use sound engineering judgement in determining whether a system is open or closed.
This is the 18th in a series that provides practical insights into on-the-job problems. To read more, visit the series hub at https://www.thechemicalengineer.com/tags/rules-of-thumb.
Disclaimer: This article is provided for guidance alone. Expert engineering advice should be sought before application.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




