Fuelling the World with Biomass
Our reliance on gasoline, diesel, and jet fuel shows little sign of declining despite the push to net zero. Could replacing crude oil in hydrocarbon liquids with cellulosic biomass feedstocks provide the solution?
HYDROCARBON liquids (gasoline, diesel, and jet fuel) make modern society possible. Hydrocarbon liquids are low-cost, dense energy carriers that are cheap to store and transport. A single gallon (3.6 L) of gasoline provides work equivalent to more than 400 hours of human labour. Hydrocarbon liquids are also feedstocks for much of the chemical industry. A third of global energy demand is met from crude oil that is primarily delivered to the final customer as hydrocarbon liquids. These liquids are also made in smaller quantities from coal, natural gas, and biomass. If crude oil had never existed, we would probably still have invented gasoline, diesel, and jet fuel because of their remarkable and useful properties.

However, the challenges of climate change1 mean we must reduce atmospheric carbon dioxide levels. Some propose we invent and deploy fossil fuel substitutes such as batteries, but, in the real world that is a century-long task – perhaps, even an impossible one. A much faster transition to low-carbon fuels requires using existing infrastructure, including oil refineries, to produce gasoline, diesel, and jet fuel from non-fossil fuel sources.
There are only two non-fossil sources of carbon for hydrocarbon liquids, the first of which is plant biomass. Plants remove CO2 from the atmosphere and convert it to biomass. Converting biomass carbon to liquid fuels and burning those fuels results in no net addition of greenhouse gases to the atmosphere. The other option is to obtain CO2 from the atmosphere and convert it into liquid fuels: so-called “electric fuels”.
Why cellulosic hydrocarbon biofuels?
Hydrocarbons typically contain chains, or rings of carbon atoms with around two hydrogen atoms per carbon atom. If we start with CO2 as the carbon source, the electric fuels option, it will take six hydrogens to make the hydrocarbon – four to remove the oxygen as water and two to add to the carbon atom.
The biomass option is lignocellulosic biomass, a wide variety of non-edible plant material. The existing biofuels industry is built on sugars, plant oils, and carbohydrates, but this resource base is limited and these feedstocks are also human food. Cellulosic biomass ((C6H10O5) n ) is a polymer for which only two hydrogens – the most expensive ingredient in these fuels – are needed to convert each carbon into a hydrocarbon, again with the oxygen leaving as water.
With this logic in mind, we conducted a series of workshops and studies on how to replace all crude oil with cellulosic biomass feedstocksto produce liquid hydrocarbons. The three Zoom workshops (due to Covid-19 restrictions) had 174 participants, including 65 from industry, 40 from national laboratories, 39 from universities and eight from government, with the broad participation and different perspectives converting an ideal into a solid pathway.
The traditional perspective is that we can’t replace all crude oil with biofuels because there is insufficient biomass. In the US, the billion-ton biomass inventory report suggests that at most, a third of the US crude oil could be replaced with biofuels without major impacts on food and fibre prices. However, that report assumes biomass provides a) the heat for the refinery, b) the carbon and hydrogen for the fuel and c) a means of removing oxygen from the biomass. If that strategy is used, a large fraction of the biomass carbon leaves biofuels plants as CO2, not as hydrocarbon fuel. With massive inputs of hydrogen and heat at the refinery from a nuclear plant, there is sufficient carbon in cellulosic biomass sources for the US to produce 25 million barrels of hydrocarbons per day. The US consumes about 18 million barrels per day. Globally, there is sufficient cellulosic carbon to replace all crude oil.
We do need massive amounts of hydrogen, though. For the next several decades, the low-cost source of hydrogen is probably natural gas with sequestration of the byproduct CO2. Depending upon assumptions, a quarter to half of US natural gas consumption would be required to produce that hydrogen. Largescale natural gas to hydrogen plants with underground sequestration of the CO2 are planned, including the ExxonMobil facility in Texas which will produce a billion cubic feet of hydrogen per day. Recent studies indicate that with a well-designed system, the climate impact of hydrogen from natural gas with carbon capture and storage (CCS) approaches that of hydrogen from electrolysis using wind or solar. There are large, embedded CO2 emissions from building wind and solar systems coupled to hydrogen production systems relative to other hydrogen production technologies.
Therefore, it is important to understand the economics of CCS. Carbon capture is expensive, while sequestration within natural gas to hydrogen systems is relatively inexpensive because the CO2 is concentrated at high pressure. Coupling CCS to a coal plant, meanwhile, is costly because the stack gas is at atmospheric pressure and is only 10% CO2. However, there are two chemical processes in which the process chemistry results in nearly pure CO2 exiting the process: conversion of natural gas to hydrogen and fermentation to produce ethanol. It is no accident that in the US, with incentives for carbon sequestration, there is a rush to build pipelines to sequester CO2 from ethanol plants and that the first chemical process to be decarbonised will be hydrogen production.
Our workshops indicate we can replace all crude oil via this strategy. The economics are different than for crude oil. Assuming hydrogen to be US$2/kg, hydrogen is the largest part of the cost of the final product. Cellulosic biomass is the second largest cost. Thus, unlike conventional biofuels, one can pay more for the biomass without major impacts on the final cost of gasoline, diesel, and jet fuel. Studies show that paying more for cellulosic biomass results in massive increases in biomass supplies from agriculture and forests.

For example, in the US, a potentially large source (~100 million tons per year) of cellulosic biomass is corn stover – the plant minus the corn grain. Corn yields in the last 100 years have gone from 20 to 180 bushels per acre with corn stover production increasing proportional to corn yields. Increasing stover prices creates incentives that simultaneously boost yields of corn and stover. In addition, much of the US and the world could produce two crops a year – corn or soybeans as the traditional food/feed crops plus a second cellulosic or “energy double crop” such as winter rye. At one time there was extensive double cropping to produce forage for farm horses, but when tractors appeared, the horses and the double cropping disappeared because it was unprofitable. Efforts to reduce wildfires in North America and Asia may also provide massive added cellulosic biomass from forest thinning to reduce the fire load in these forests. Preliminary estimates are that a largescale system could produce bio-crude at somewhere between US$70 to US$80/barrel.
System design
A system design with three major components was developed to replace all crude oil (Figure 1). The components are: the refinery, the depots that convert local low-density biomass into an economically shippable commodity, and the hydrogen/heat production systems.
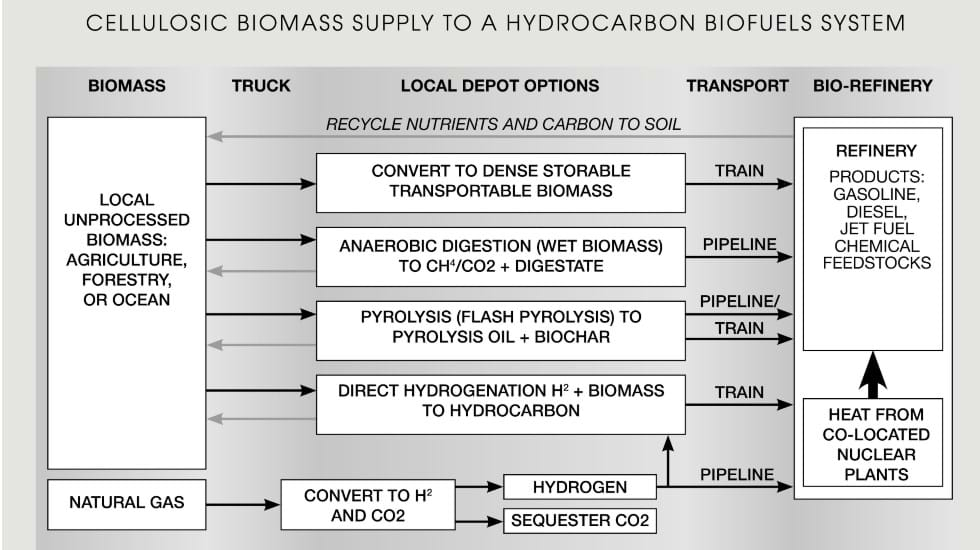
The central component is the large integrated bio-refinery. These are existing conventional oil refineries with front-end modifications. Globally, large integrated oil refineries have replaced small refineries for three reasons. Firstly, there is the traditional economics-of-scale of large chemical plants. Secondly, large refineries can blend different grades of crude oil to match their refining capabilities. This feature lowers the average cost of crude oil to large refineries relative to small refineries. Finally, large integrated refineries increase refinery revenues by rearranging molecules and thereby vary the products to match changing seasonal market demand. The same logic applies to producing liquid hydrocarbon biofuels: existing refineries with modified front ends will produce liquid hydrocarbons. Under this model, refineries would incrementally convert from primarily crude oil to bio crude oils as feedstocks – something that is beginning to happen on a small scale. This approach accelerates the transition away from crude oil. The costliest input is hydrogen (delivered via pipeline).
The second input is cellulosic biomass. However, the density of raw biomass is low. It can’t be shipped economically more than 50 to 80 km. Local processing depots are required to convert raw biomass into high-density, storable, commodities for easy shipment to the refinery. The depots serve other functions to increase farm revenue. For example, in plants such as alfalfa, the protein is in the leaves, not the stems. Separation processes at the depots can produce protein-rich animal foods and other products, with the less valuable cellulosic biomass (stems) used for fuels production. The choice of depot depends upon the characteristics of biomass feedstock and refinery economics. There are multiple depot options:
- Biomass densification to dry pellets and shipment to refineries by truck, train, barge, or ship: Raw biomass has a density less than about 50 kg per m3. Densification increases the density by a factor of ten or more. Biomass pelleting is a commercial process. The DRAX power plant in the UK burns more than 7m tons of pelletised cellulosic biomass per year and most of that pelleted biomass is imported from the US. But while DRAX and other power stations demonstrate long-distance biomass transport, burning biomass is the lowest-value use in a world short of liquid biofuels and does not recycle soil nutrients, as discussed below. In fact, in the view of the authors who, as chemical engineers, think feedstock first, biomass purely as an energy source should be left to isolated locations, campfires, and fireplaces where it provides heat and enjoyment
- Anaerobic digesters to produce (1) a methane/carbon dioxide gas mixture shipped via pipeline to the refinery and (2) a carbon and nutrient-rich digestate that is returned to the soil: In anaerobic digestion, the feed is an aqueous slurry; thus, this becomes the preferred process for biomass if it has a high water content to avoid the costs of drying the biomass. Experience shows that recycling of this digestate improves long-term soil fertility and carbon content. This process produces renewable natural gas and is commercial in some parts of the world for some types of biomass
- Flash heating of biomass to produce pyrolysis (bio) oil and bio char: Dry biomass is rapidly heated to about 500°C, producing a liquid bio crude oil, a char, and gases. The bio char can be recycled to the soil to improve soil productivity or used as a feedstock for hydrocarbon fuels production. The process is commercial on a small scale
- Direct hydrogenation of biomass: This option requires large hydrogen inputs – most likely supplied via pipeline
There are many ways to convert biomass into bio crude oil. The local feedstock and the relative economics of depots, transportation, and the refinery will determine where and how the conversion process occurs. The processes include flash heating, direct hydrogenation of biomass, and Fischer-Tropsch gasification of the biomass to a mixture of carbon monoxide and hydrogen that is then converted into liquid hydrocarbons. Flash heating and Fischer-Tropsch are existing, largescale commercial processes.
Direct hydrogenation is used to upgrade many types of heavy crude oils and remove sulfur. Oxygen is chemically similar to sulfur (both are column six elements in the periodic table); thus, most hydrogenation processes that remove sulfur also remove oxygen as water. The cellulosic material is mixed with a hydrocarbon solvent and hydrogen at several hundred degrees C over a catalyst. There has been extensive work on direct hydrogenation of other feedstocks including coal, but limited work on direct hydrogenation of cellulosic biomass. Given the success of upgrading crude oil, we believe that direct hydrogenation in the long term is likely to become the dominant process at the biorefinery.

Today’s large refineries have heat inputs of gigawatts and consume the equivalent of about 10% of the incoming crude oil in furnaces and boilers to operate. The processing of biomass will bring associated water into the refinery, thereby increasing internal heat consumption. In the long term, co-located nuclear reactors provide the necessary gigawatts of heat for refinery operations to minimise consumption of biomass per unit of product while avoiding CO2 releases to the atmosphere. Heat can be provided by high-temperature nuclear reactors like the recently announced plans by Dow and X-energy to add nuclear reactors for process heat at the Dow Seadrift site in Texas.
Carbon sequestration and long-term soil productivity
Properly designed, this system can remove massive amounts of CO2. Two of the depot-processing options produce refractory carbon (via anaerobic digestate and pyrolysis char) that is recycled to the soil, thereby sequestering carbon in the soil, improving soil properties and recycling inorganic nutrients such as potassium and phosphorous.
There are two other mechanisms for negative emissions. Anaerobic digestion produces a methane/CO2 mixture. All the carbon in these mixtures can be converted into liquid fuels with the addition of hydrogen. Alternatively, the CO2 can be separated out and sequestered underground while the methane is converted into liquid fuels. Many refinery processes can produce variable amounts of CO2 and hydrocarbons depending upon hydrogen addition. Assuming a market for sequestered carbon, variable sequestration of carbon would stabilise hydrocarbon fuel and biomass prices against variations in biomass production and biofuels demand.
To produce liquid hydrocarbons, we only want carbon and hydrogen – not the other elements in biomass including nitrogen, potassium, and phosphorus, etc. This is unlike production of food or fibre where we need the other nutrients for human and animal health. The refinery and depots enable recycling of nutrients in digestate and bio char back to farms and forests to improve long-term soil productivity. Bio char and digestate from anaerobic digestion improve the properties of soil including water retention, while sequestering carbon, thereby enabling long-term sustainable agriculture and forestry.2 The simplistic perspective that biofuels competes with food and fibre production is false – it depends upon how liquid biofuels are produced.
There are two large competing uses for cellulosic biomass: (1) fuel use in industrial and utility boilers and (2) production of renewable natural gas – methane. There is insufficient biomass to replace crude oil if we use biomass primarily as an energy source rather than as a carbon source for liquid hydrocarbons. The choice to replace all crude oil partly depends upon economics and partly upon policies, including incentives for biomass use.
Observations and conclusions
If we want to decarbonise quickly at an affordable cost, we must use existing infrastructure and technology where possible. Replacing crude oil with cellulosic feedstocks to produce liquid hydrocarbon fuels, with their remarkable properties, is a massive challenge – but it pales in comparison to quickly inventing, developing, and deploying alternative technologies to replace liquid hydrocarbons. Independently, nobody has found any chemical fuel that comes close to liquid hydrocarbons in terms of energy density and safety. Because of that physical reality, no one should be surprised if a thousand years from now we are still using liquid hydrocarbon biofuels and feedstocks, long after crude oil has disappeared.
References
- CW Forsberg, BE Dale, DS Jones, T Hossain, ARC Morais and LM Wendt, Replacing Liquid Fossil Fuels and Hydrocarbon Chemical Feedstocks with Liquid Biofuels from Large-Scale Nuclear Biorefineries, Applied Energy, 298, 117525, 15 September 2021
- L Schulte Moore and J Jordahl, editors, Carbon Science for Carbon Markets: Emerging Opportunities in Iowa, CROP 3175, Iowa State University Extension and Outreach, Ames, Iowa, 2022
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




