Energy Saviours: Part 3
Tom Baxter shares more options for chemical engineers to boost energy efficiencies
AS STATED in my previous articles in The Chemical Engineer, I believe chemical engineers have a hugely significant role to play in decarbonising the environment and reducing other harmful gaseous emissions. Amongst the mix of decarbonisation options, energy efficiency is a key enabler. This article builds upon “Energy Saviours” parts 1 and 2, and discusses further options and considerations for energy efficiency.
Energy Recovery from Low Grade Heat
The Rankine Cycle
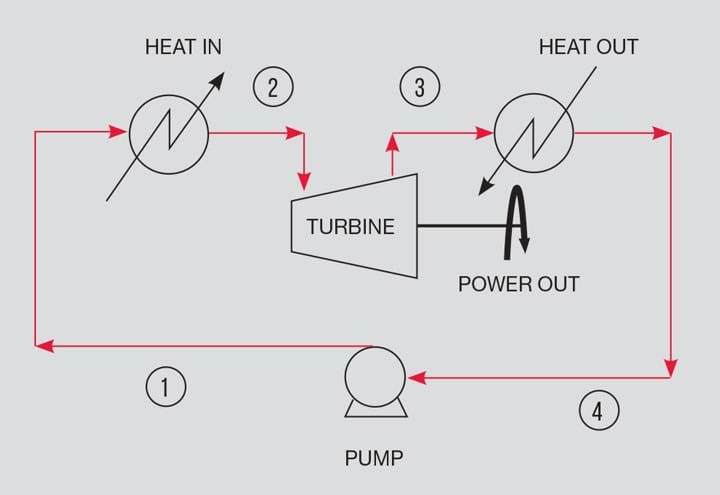
The Rankine cycle makes use of a temperature difference to generate power. The ideal cycle is in four parts. The hot source is used to vapourise a liquid at constant pressure. The vapour is used to drive a turbine to isentropically extract work. The vapour is condensed to a liquid utilising the cold fluid at constant pressure. The liquid is returned to the vapouriser using a pump. It is the principle adopted in heat recovery steam generation systems in combined cycle power stations.
The cycle is shown in Figures 1 and 2. Figure 2 shows the Rankine path on a pressure/enthalpy plot. The plot includes isotherms (green dash) and lines of constant entropy (black dash). Starting from 1, the fluid is heated to 2 using a hot source.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




