A Clean Bill of Health
OBSERVING some large tubular heat exchangers during a university vacation course at an oil refinery in the late 1960s, I asked how often they were cleaned and was told “once a year during the annual shutdown”. When a saucepan of milk is heated on a cooker, it takes a couple of minutes for it to boil, and when poured there is a deposit on the bottom of the pan, a mixture of protein, fat, sugars and minerals. Both examples are a result of fouling, albeit with quite different timescales and both require cleaning to bring the surfaces back to their original condition.
There are two main types of fouling within the food industry, those caused by heat, and those that are unrelated to heat. Fouling of equipment such as heat exchangers can result in both a loss of heat transfer performance and an increase in pressure drop. At the other end of the temperature scale, freezing can also create fouling. For example, prior to freezing, peas are washed and then blanched to inactivate the enzymes that degrade the peas during frozen storage. They are then cooled with a water spray and passed across a vibrating screen to remove residual water. However, a significant amount of moisture remains, and freezes within the freezer, which then requires defrosting every few hours.
Other areas where cleaning may be critical include prior to a production shutdown or a product changeover. This is particularly important where both allergen and allergen-free products are produced on the same line. Dried soups, for example, may have many ingredients of both types and the cleaning regime applied will vary depending on the risk of the product changeover. If an allergen-containing product is followed by another allergen product the risk is low, similarly if allergen-free follows allergen-free. The highest risk occurs when an allergen product is followed by one that is allergen-free, thus requiring a far more rigorous cleaning regime.
Cleaning may also be required for microbiological reasons, for example where a product has been pasteurised and hence a residual level of organisms remains. During a production run there may be an increase in total numbers in the final product and to ensure the numbers are kept within specification, regular cleaning will be required. In the 1980s, a typical frozen food line would run for two shifts, ie 16 hours, and cleaning would take place during the night shift. This resulted in a low production efficiency of 66% and by improving the design, operation and cleaning of the line, run lengths of 60 hours with 4 hours cleaning could be achieved without compromising safety, a production efficiency of over 90%. It is therefore essential to understand the challenges posed by the production processes so that the appropriate cleaning schedules can be specified.
A necessary evil?
For the factory production team, cleaning is usually seen as “a necessary evil” because it is:
- non-productive, resulting in plant downtime;
- time consuming;
- poorly understood – “out of sight, out of mind”;
- aggressive – cleaning regimes, for example, use hot highly alkaline/acids, as well as biocides;
- corrosive, hence the potential for high maintenance costs; and
- subject to safety issues, both for personnel and equipment.
However, cleaning is essential for food quality, safety and peace of mind.
Approaches to cleaning will depend on the type of equipment, whether open or closed, and particularly the ease of access to the product contact surfaces (see Table 1).
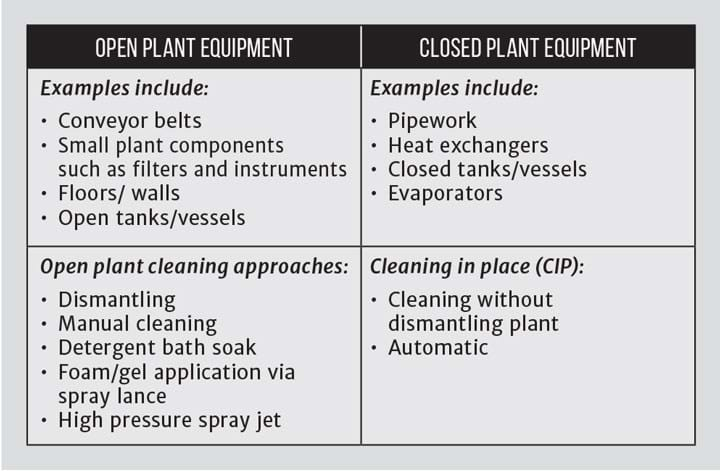
Cleaning in place
The following examples will focus mainly on cleaning in place, or CIP, although the principles are relevant for open plant cleaning with both involving a combination of mechanical and chemical forces to achieve the desired end result. Cleaning in place is the circulation of chemicals and/or water through plant that remains assembled as for production. All product contact surfaces are cleaned and where necessary disinfected or sterilised to an
acceptably high and consistently reproducible standard.
The main stages in a CIP process are:
- Product recovery/rinsing – removal of residual product from the line or equipment usually using warm water and where practical, recovering as much product as possible to minimise effluent.
- Cleaning – Removal of any remaining product deposits from the equipment surfaces by circulating detergents through the plant. After the cleaning stage, the detergent will usually be recovered.
- Final rinse with potable water – Removal of chemicals from the line with potable quality water.
- Disinfection/sterilisation – Reduction (disinfection) or elimination (sterilisation) of microorganisms in the plant to ensure the appropriate hygiene standards are maintained.
The key factors involved are time; detergents and biocides; temperature; and flowrate.
TIME
The time required for effective cleaning will depend on the process and how heavily the line or equipment is fouled. The aim is to minimise time, and maximise production efficiency at an acceptable cost.
DETERGENT AND BIOCIDES
An effective detergent will be able to wet, penetrate, and react with the soil. The reaction products must then avoid redeposition onto the cleaned surface. Detergents are usually a combination of chemicals such as surfactants to reduce surface tension and enhance penetration into the soil. Inorganics such as sodium hydroxide are widely used to remove the organic components of the soil – such as proteins and fats – being effective and relatively low cost, although it does not have good rinsing characteristics. It is usually circulated as a first stage in the cleaning process followed by a rinse and then an acid step to remove the mineral components such as calcium salts. For this step, inorganic acids such as nitric, phosphoric or a combination of the two are typically used. In some cases, single-stage detergents are available, formulated to remove both organic and inorganic soils in the same step and thus save time.1
Biocides are strong oxidising chemicals such as sodium hypochlorite or peracetic acid, the former being potentially corrosive to stainless steel, the latter being increasingly widely used. Concentrations of 100–250 mg/L are typical for disinfection with an order of magnitude increase required for sterilisation.
TEMPERATURE
Increasing temperature will increase the rate of reaction between detergent and soil. However, care must be taken to ensure that this does not have an adverse effect on the equipment, such as hardening of gaskets, which may lead to leakage. For disinfection, temperatures of 70–90oC are typical with 120–140oC used for sterilisation.
FLOWRATE (FLUID VELOCITY)
Flowrate or fluid velocity is key in delivering the detergent to the soil on the product contact surfaces, as well as removing the reaction products back into the bulk solution. Milk fouling studies using a plate heat exchanger have shown the strong influence of fluid velocity on the time to clean1, with time required decreasing significantly as the velocity was increased from 0.1 to around 1.5 m/s, beyond which there was little or no additional benefit. The rapid reduction in cleaning time suggested that the boundary layer thickness and the diffusion of the detergent into the fouling deposits was the rate-controlling step. However, as the velocity increased above 1.5 m/s, the reaction rate between detergent and soil became the rate-controlling step.
The 1.5 m/s figure is widely recommended as the optimum cleaning velocity. However, it may be unachievable in practice due to, for example, an excessive pressure drop. A more practical approach is to maximise the velocity during cleaning, as even a modest increase from 0.2 to 0.3 m/s will be beneficial.2 The original work1 was carried out each day at the same time, with fresh milk from a small local dairy herd, anticipating that this would minimise day-to-day variations in fouling. However, this was not the case and highlighted the variable behaviour of complex food fluids, such as milk, when heated.
The study also evaluated the effect of detergent concentration on cleaning time and unexpectedly showed that there was an optimum concentration of sodium hydroxide, although the effect was not as pronounced as for fluid velocity. The usual approach for difficult soils was to increase the detergent concentration but this study suggested that reducing detergent concentration should also be evaluated when trying to optimise cleaning. One factory investigation I took part in resulted in the detergent concentration being reduced by half without any adverse effect on cleaning performance. However, it was still impossible to persuade the client that cleaning the process water tanks weekly for 2 hours with 80oC nitric acid was excessive and risked corrosion.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




