US-designed natural gas reactor heats up alternative propylene production

US RESEARCHERS have designed a reactor that produces propylene from natural gas, potentially providing a sustainable pathway to meeting the growing global demand for the popular monomer.
Propylene can be used to make polypropylene, found in everyday products such as carpets, cold-weather clothing, and food packaging. It is traditionally produced as a byproduct of petroleum refining and from ethylene production by steam cracking hydrocarbon feedstocks.
The reactor, designed by chemical engineers at the University of Michigan, takes propane from shale gas, and converts it into propylene and hydrogen using a process called propane dehydrogenation.
Demand for oil is expected to slow significantly by 2028, yet shale gas production in the US is increasing and expected to become half of the US natural gas supply by 2040.
Research into shale gas and fracking have found that it contributes to air pollution. However, as a natural gas, it has lower CO2 emissions than other fuels thanks to its higher energy content. It could also help the US with energy security, providing an alternative to oil and gas imports.
The issues with propane dehydrogenation
Though there is an abundance of propane in shale gas, it has proved difficult to convert to propylene due to the heat required for the reaction, as Suljo Linic, a member of the research group, explains: “You need to heat that reaction to drive it, and standard methods require extremely high temperatures to produce enough propylene.
“At those temperatures, you do not just get propylene but solid carbon deposits and other undesirable products that impair the catalyst. To regenerate the reactor, we need to burn off the solid carbon deposits often, which makes the process inefficient.”
What does this reactor do?
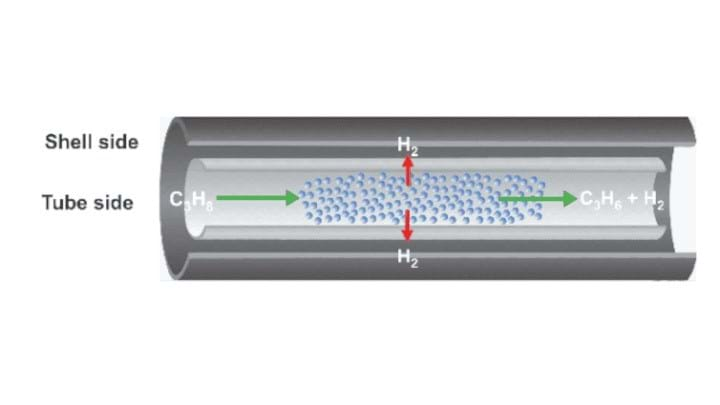
The University of Michigan-designed reactor uses a catalyst-membrane system. This contains two layers, separating the hydrogen to an outer chamber and isolating the propylene in an inner tube.
The hydrogen is controllably burned in the chamber by adding oxygen and forming water. This produces heat that can speed up the dehydrogenation process.
Researchers say that because heat is generated from burning hydrogen inside the reactor, this method would allow propylene production plants to produce natural gas-based propylene without installing extra heaters.
They estimate that a plant producing 500,000 t/y of propylene could save around US$23.5m, as well as additional operational savings from burning hydrogen produced in reaction, rather than other fuels.
The team is now working with the University of Michigan’s Innovation Partnerships research community to patent the reactor and take it to market.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




