New plastic can be recycled repeatedly
SCIENTISTS at the US Department of Energy’s (DOE) Lawrence Berkley National Laboratory (LBNL) have developed a next-generation plastic that can be recycled repeatedly without loss of performance or quality.
Plastics can contain additives to make them fit for purpose, and in many plastics these additives stay tightly bound to monomers even after processing in recycling plants. Because plastics are recycled in a mix of plastics, that contain various additives to achieve various properties, the properties of the new material are unknown and difficult to predict. Very few plastics can be recycled without loss of performance or aesthetics.
The multidisciplinary team at LBNL has developed a new plastic, polydiketoenamine (PDK), which can be recycled without losing performance or quality, and can be reformulated with different properties.
The team achieved synthesis of PDKs by mechanically grinding triketone monomers with amines, using a ball mill. This synthesis is possible because triketones and amines react spontaneously. Additives were used in synthesis to achieve different properties, such as colour.
Recycling was achieved by first depolymerising PDK using a strong aqueous acid. In experiments, at room temperature, 5.0 M sulfuric acid achieved complete depolymerisation in less than 12 hours. 5.0 M hydrochloric acid was similarly effective. The time lapse video above (courtesy of Peter Christensen, Lawrence Berkeley National Laboratory) shows the degradation of a piece of PDK plastic into its monomers. In the acid, the monomers are able to dissociate from each other and additives because they are soluble at different pH levels.
Depolymerisation yielded solid material – triketone monomers and any additives which may have been used – and amines in an aqueous solution. Triketones were recovered by first filtering the solid from the aqueous solution. For complex solutions triketone, monomers were separated from additives by extracting the solid into aqueous base which dissolved the triketones. The insoluble compounds could then be filtered out and the triketone could be precipitated from solution by reacidification. The amine component was recovered from aqueous solution using an ion exchange process.
In addition to additives, the researchers found that the recovery process allows triketone and amine monomers to be recovered from mixed plastic waste. This is because conventional plastics such as PET, which were not made to be recycled, do not depolymerise under the same conditions.
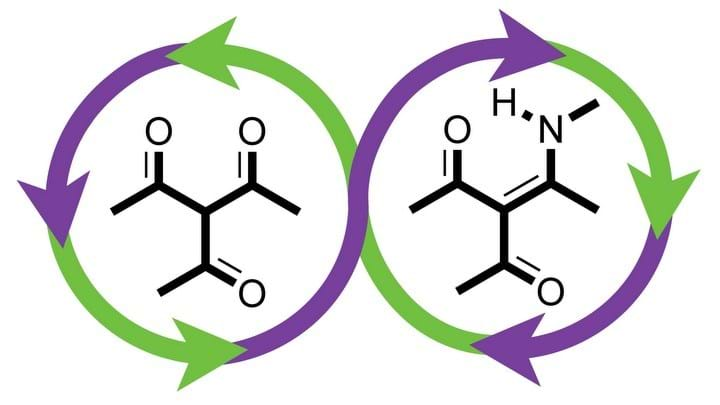
The recovery process allowed the researchers to obtain pure triketone and amine monomers, at yields of more than 90%. Research leader Brett Helms said that “PDKs challenge the status quo by offering chemical circularity, where monomers can be recovered in high yield and purity after depolymerisation in acid”.
In additional to recycling, PDKs could also be upcycled, ie used to formulate new materials from recycled material, where the new material has a higher value application.
According to Helms, recycled PDK polymer has characteristics nearly identical to virgin PDK, and the researchers have not yet seen a limit to the number of cycles PDK can go through without losing performance.
PDKs represent an opportunity to make plastics’ life cycles more circular, as they could incentivise recovery and reuse of plastics, diverting them from landfills and oceans. They could provide a good alternative to currently used nonrecyclable plastics. According to Helms, PDKs could replace plastics such as nylons and polyurethanes.
The researchers plan to develop PDK plastics with a wide range of thermal and mechanical properties for diverse applications. Helms said that the group is “advancing the PDK platforms in the area of reversible adhesives, elastomers, packaging, and textiles”.
According to Geyer et al 2017, as of 2015, only 9% of all plastic waste had been recycled. 12% had been incinerated and the rest ended up in landfills, dumps, and the environment. Land-based plastic waste contributes more than 80% of the plastic waste found in oceans, says a report from the Ocean Conservancy, which works to protect the ocean.
Helms said: “We’re at a critical point where we need to think about the infrastructure needed to modernise recycling facilities for future waste sorting and processing.”
“If these facilities were designed to recycle or upcycle PDK and related plastics, then we would be able to more effectively divert plastic from landfills and the oceans. This is an exciting time to start thinking about how to design both materials and recycling facilities to enable circular plastics.”
PDK technology is currently available for licensing and collaboration. According to Helms the researchers are in ongoing licensing discussions with “major players in plastics throughout the world”.
The research was conducted at LBNL’s Molecular Foundry and was supported by the DOE’s Laboratory Directed Research and Development Program.
Nature: http://doi.org/gf27v3
Correction
This article was edited on 28 September 2020 to change “only 9% of all plastic had been recycled” to instead read “only 9% of all plastic waste had been recycled”, and to credit Geyer et al 2017 and Ocean Conservancy.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




