The Problem Solver

Solving a serious environmental problem on a maleic anhydride plant
Natal Coast, South Africa, 1983
A COMPANY called National Chemical Products (a subsidiary of Sentrachem at the time) installed a maleic anhydride (MA) plant on its site on the beautiful South Coast of Natal.
This article looks at how we solved a serious environmental problem at the plant, revolving around a mysterious pool of acidic effluent.
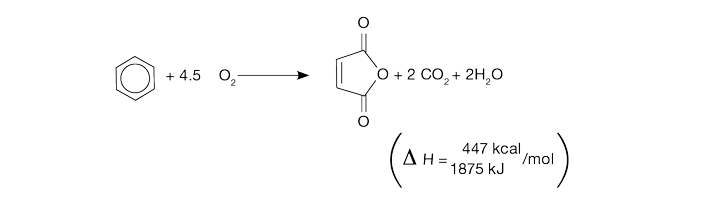
Maleic anhydride
Maleic anhydride is produced by the following chemistry, a highly exothermic process:
A simplified flowsheet of the process is given below:

The large amount of excess heat was used to generate steam, of which there was an abundant supply. Note that there was a scrubber to remove particles of MA from the vent gas.
The plant was sited near the coast, and the authorities granted a licence to operate the plant, providing that no liquid effluent was produced, which would be harmful to aquatic life. However, although the plant was designed to produce no effluent, in practice an acidic liquid effluent was produced from day 1. When I first visited the plant, there was a “mini-lagoon” of liquid effluent, with a small trickle of acidic liquid flowing into it continuously.
This was a serious embarrassment to the plant management, who were under considerable pressure to solve the problem.
However, the envronmental problem turned out to be very difficult to solve. Finding the source of the liquid was a major problem. There were no flow indicators to show what the rate of flow was, and the source of the liquid was a mystery. MA has a melting point of around 35°C, so liquid MA was transported around the plant in piping with hot water jackets. Earlier investigators homed in on the idea that the source of the liquid was a leak on one of the jackets.
Finding a jacket leak was very difficult, because the jackets were of 316 L stainless steel, so it was not possible to see through them. Earlier investigators decided that the only way to stop the leaks was to replace every millimeter of jacketed piping. Since this work could only be done during a shutdown, and since it is very expensive to replace all of the piping, this effort had been going on for years.
Each “new” chemical engineer was introduced to this problem in the hopes that their new insights would make the problem go away. I was no exception. I started by getting a copy of the supplier’s mass balance, and started working through their documents. Their design calculations were set out clearly, so it did not take very long to check them.
The Eureka moment
The calculations which got the most interest from me were those of the scrubbing system, since that was a potential source of the liquid effluent. I went over them repeatedly, and they seemed to be clear and water-tight. However, staring at these calculations for the umpteenth time, I suddenly realised that they contained a serious flaw. The scrubber was fed with water, to dissolve the MA vapour which flowed into it. This formed a solution of MA in water. The calculation assumed that the solution in the top of the scrubber was pure water, ie that the vapour pressure of water in the solution could be calculated from steam tables. As a result, they calculated that far more water vapour would flow out into the atmosphere than actually did. When I recalculated the amount of water escaping into the atmosphere based on an MA solution, much less water would be evaporated!
I recalculated the water loss if the solution was MA, and what temperature would be needed in the scrubber to ensure complete evaporation. This was just over 28°C. We then found a redundant plate heat exchanger, and connected it up to the water feed into the scrubber. We set up a control system, so that we could set the water temperature, heated by waste steam.
We set the water temperature at 28.5°C and watched the effluent pipe. As the temperature rose, the water trickle reduced, until at 28.5°C the water flow stopped. We were thrilled; the problem was solved! A few days later, I received a nice letter from the managing director, complimenting us on the result. He was especially pleased that the extra equipment only cost about £300.
When a stranger arrives and solves a major problem without seeming to even break sweat, the attitude of the plant personnel is mixed, at best
At the time, in spite of the fact that our solution to the problem was a major breakthrough and saved tens of thousands of pounds a year, the response by most of the staff was luke-warm at best. I found this puzzling. However, with hindsight, I now understand it, especially after more experience as a consultant.
When a stranger arrives and solves a major problem without seeming to even break sweat, the attitude of the plant personnel is mixed, at best. Some people are afraid that the boss will question their abilities by asking the question “ How come you people didn’t spot it first?” Some people question their own ability to solve problems. They forget that the outsider may have special experience which makes some problems easier to solve. By the time I had been working there for a while, they started to trust me and things got easier.
To read more of Jimmy Hunter's Chemeng Chronicles, visit the series hub.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.




